Chemistry:Chlorosyl fluoride
From HandWiki
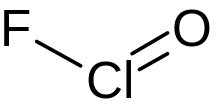
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| ClFO | |
| Molar mass | 70.45 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chlorosyl fluoride is an inorganic compound of chlorine, fluorine, and oxygen with the chemical formula OClF.[1][2][3]
Synthesis
- Partial hydrolysis of chlorine trifluoride in diluted gas phase at low temperatures.[4]
- Reaction of chlorine trifluoride with nitric acid.[4]
Chemical properties
Chlorosyl fluoride is thermolabile and disproportionates to ClF and ClO2F.[4]:
- 2FClO → ClF + FClO
2 - 2FClO → 2ClF +O
2
- 2FClO → ClF + FClO
References
- ↑ Müller, Holger S. P (10 December 1999). "Infrared spectroscopy and molecular properties of chlorosyl fluoride, FClO" (in en). Chemical Physics Letters 314 (5): 396–402. doi:10.1016/S0009-2614(99)01197-5. ISSN 0009-2614. Bibcode: 1999CPL...314..396M. https://www.sciencedirect.com/science/article/abs/pii/S0009261499011975. Retrieved 27 March 2023.
- ↑ Müller, Holger S. P.; Cohen, Edward A. (8 February 2002). "The molecular properties of chlorosyl fluoride, FClO, as determined from the ground-state rotational spectrum". The Journal of Chemical Physics 116 (6): 2407–2416. doi:10.1063/1.1433002. ISSN 0021-9606. Bibcode: 2002JChPh.116.2407M. https://aip.scitation.org/doi/abs/10.1063/1.1433002?journalCode=jcp. Retrieved 27 March 2023.
- ↑ Vogt, J. (2011). "755 ClFO Chlorosyl fluoride". Asymmetric Top Molecules. Part 3. Landolt-Börnstein - Group II Molecules and Radicals (Springer Berlin Heidelberg) 29D3: 296–298. doi:10.1007/978-3-642-14145-4_177. ISBN 978-3-642-14144-7. Bibcode: 2011LanB.29D3..296V. https://www.researchgate.net/publication/258535120.
- ↑ 4.0 4.1 4.2 Haupt, Axel (22 March 2021) (in en). Organic and Inorganic Fluorine Chemistry: Methods and Applications. Walter de Gruyter GmbH & Co KG. p. 139. ISBN 978-3-11-065933-7. https://books.google.com/books?id=5fwkEAAAQBAJ&dq=Chlorosyl+fluoride&pg=PA618. Retrieved 27 March 2023.
 |

