Chemistry:Iodyl fluoride
From HandWiki
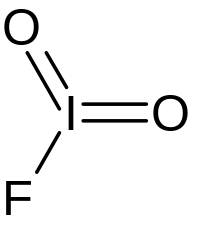
| |
| Names | |
|---|---|
| IUPAC name
Fluoro(dioxo)-λ5-iodane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| FIO2 | |
| Molar mass | 177.901 g·mol−1 |
| Appearance | colorless crystals |
| Density | 4.982 g/cm3 |
| Melting point | 200 °C (392 °F; 473 K) |
| Reacts with water | |
| Related compounds | |
Related compounds
|
Iodosyl pentafluoride Iodosyl trifluoride Periodyl fluoride Iodyl trifluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Iodyl fluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IO
2F. The compound was initially synthesized in 1951.[1]
Synthesis
- Iodyl fluoride can be decomposed by iodosyl trifluoride heated to 110 °C in nitrogen. Since this reaction is reversible, the reaction requires constant removal of iodine pentafluoride.[1]
- 2IOF
3 ⇌ IO
2F + IF
5
- 2IOF
- Dissolving the anhydride of iodic acid, I
2O
5, in anhydrous hydrofluoric acid.[2]
- I
2O
5 + HF → IO
2F + HIO
3
- I
Physical properties
Iodyl fluoride forms colorless crystals of orthorhombic system.[3] Reacts with water.[4]
Chemical properties
Iodyl fluoride is stable in dry air, but slowly hydrolyzes to iodic and hydrofluoric acids in moisture.[1]
- IO
2F + H
2O → HIO
3 + HF
- IO
The compound reacts with strong fluorinating agents such as bromine trifluoride and selenium tetrafluoride to form iodine pentafluoride. Iodyl fluoride can be reduced to elemental iodine by pure hydrogen peroxide.[5][6]
- 3IO
2F + 4BrF
3 → 3IF
5 + 2Br
2 + 3O
2 - IO
2F + 2SeF
4 → IF
5 + 2SeOF
2
- 3IO
References
- ↑ 1.0 1.1 1.2 Aynsley, E. E.; Nichols, R.; Robinson, P. L. (1 January 1953). "126. Reactions of iodine pentafluoride with inorganic substances. Iodine oxytrifluoride and iodyl fluoride" (in en). Journal of the Chemical Society: 623–626. doi:10.1039/JR9530000623. ISSN 0368-1769. https://pubs.rsc.org/en/content/articlelanding/1953/JR/jr9530000623. Retrieved 24 May 2023.
- ↑ Wiberg, Egon; Wiberg, Nils (2001) (in en). Inorganic Chemistry. Academic Press. p. 468. ISBN 978-0-12-352651-9. https://books.google.com/books?id=Mtth5g59dEIC&dq=Iodosyl+trifluoride&pg=PA468. Retrieved 24 May 2023.
- ↑ Minkwitz, Rolf; Berkei, Michael; Ludwig, Ralf (1 December 2001). "Crystal Structure of IO2F" (in en). Inorganic Chemistry 40 (25): 6493–6495. doi:10.1021/ic0105462. ISSN 0020-1669. PMID 11720506. https://pubs.acs.org/doi/10.1021/ic0105462. Retrieved 24 May 2023.
- ↑ Haynes, William M. (4 June 2014) (in en). CRC Handbook of Chemistry and Physics. CRC Press. p. 4-67. ISBN 978-1-4822-0868-9. https://books.google.com/books?id=bNDMBQAAQBAJ&dq=Iodosyl+trifluoride&pg=SA4-PA67. Retrieved 24 May 2023.
- ↑ Schmeisser, M.; Brändle, K. (1 January 1963). "Oxides and Oxyfluorides of the Halogens" (in en). Advances in Inorganic Chemistry and Radiochemistry (Academic Press) 5: 41–89. doi:10.1016/S0065-2792(08)60152-1. ISBN 9780120236053. https://www.sciencedirect.com/science/article/abs/pii/S0065279208601521. Retrieved 24 May 2023.
- ↑ (in en) Advances in Inorganic Chemistry and Radiochemistry. Academic Press. 1 January 1963. ISBN 978-0-08-057854-5. https://books.google.com/books?id=pRXIwIV-hB8C&dq=%22Iodyl+fluoride%22&pg=PA83. Retrieved 24 May 2023.
 |

