Chemistry:4-Dehydroepiandrosterone
From HandWiki
| |
| |
| Names | |
|---|---|
| IUPAC name
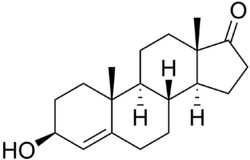
3β-Hydroxyandrost-4-en-17-one
| |
| Systematic IUPAC name
(3aS,3bR,7S,9aR,9bS,11aS)-7-Hydroxy-9a,11a-dimethyl-2,3,3a,3b,4,5,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthren-1-one | |
| Other names
Androst-4-en-3β-ol-17-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H28O2 | |
| Molar mass | 288.431 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
4-Dehydroepiandrosterone (4-DHEA) is a steroid that is an isomer of 5-dehydroepiandrosterone.
4-DHEA has been prepared by laboratory synthesis.[1][2]
Synonyms
Synonyms for 4-dehydroepiandrosterone are:
3β-Hydroxy-4-androsten-17-one, 3β-hydroxyandrost-4-en-17-one, 3β-hydroxy-D4-androsten-17-one, 3β-hydroxyandrost-4-en-17-one, 3β-hydroxy-etioallocholan-4-en-17-one, and 4-androsten-3β-ol-17-one.
References
- ↑ Klimstra, Paul D.; Colton, F. B. Synthesis of 3β-hydroxyestr-4-en-17-one and 3β-hydroxyandrost-4-en-17-one. Steroids (1967), 10(4), 411-24.
- ↑ Ward, Margaret G.; Orr, James C.; Engel, Lewis L. A convenient synthesis of 3β-hydroxyandrost-4-en-17-one. Journal of Organic Chemistry (1965), 30(5), 1421-3.
 |

