Chemistry:Benorterone
 | |
| Clinical data | |
|---|---|
| Other names | SKF-7690; FC-612; 17α-Methyl-B-nortestosterone; 17α-Methyl-B-norandrost-4-en-17β-ol-3-one |
| Routes of administration | By mouth, topical[1] |
| Drug class | Steroidal antiandrogen |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
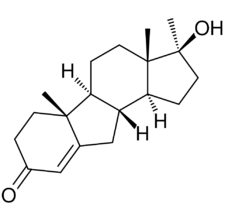
| Formula | C19H28O2 |
| Molar mass | 288.431 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Benorterone, also known by its developmental code name SKF-7690 and as 17α-methyl-B-nortestosterone, is a steroidal antiandrogen which was studied for potential medical use but was never marketed.[2][3] It was the first known antiandrogen to be studied in humans.[1] It is taken by mouth or by application to skin.[1]
Pharmacology
Pharmacodynamics
Benorterone is an antiandrogen, or an antagonist of the androgen receptor (AR), the biological target of the androgen sex hormones testosterone and dihydrotestosterone.[3] In one study, the affinity of benorterone for the AR was found to be about 5-fold greater than that of cyproterone acetate in rat prostate cytosol; the Ki values were 0.7 nM for benorterone and 3.7 nM for cyproterone acetate, which were 243% and 46% of those of testosterone (Ki = 1.7 nM), respectively.[4][5] However, another study found that benorterone had only 11% of the affinity of dihydrotestosterone for the androgen receptor.[4] Although an antiandrogen, benorterone actually is a very weak partial agonist of the AR and has been reported to possess weak androgenic activity.[6] The same is true for cyproterone acetate and other steroidal antiandrogens.[7][8]
Unlike certain other steroidal antiandrogens such as cyproterone acetate, benorterone is not also a progestogen, instead being described as a selective and pure AR antagonist similarly to nonsteroidal antiandrogens such as flutamide and bicalutamide.[9][3] However, although it is described as not being a progestogen, benorterone was found to produce "a highly variable decrease in plasma testosterone levels," indicating that it has weak antigonadotropic effects.[3][10] The reasons for this are unclear, as other pure antiandrogens such as cyproterone (not cyproterone acetate) and flutamide do not do this and instead produce consistent increases in testosterone levels.[11] However, it is notable that the anabolic steroid methyltestosterone, which benorterone differs from in chemical structure only by the removal of a carbon atom in the B ring, is aromatized into the estrogen methylestradiol and has potent estrogenic activity.[12] Estrogens are antigonadotropic similarly to androgens and progestogens and are likewise able to suppress testosterone levels.[13] In accordance, the compound corresponding to what would be the aromatized form of benorterone, 17α-methyl-B-norestradiol, has been described and has been reported to possess estrogenic activity, although the aromatization of benorterone has not been assessed.[14]
A couple of studies found that prothrombin levels decreased by 50% in some patients treated with benorterone, although a causal relationship between this change and benorterone could not be shown.[3]
Pharmacokinetics
Benorterone is active orally and topically and has been studied by both of these routes of administration.[1]
Chemistry
Benorterone, also known as 17α-methyl-B-nortestosterone or as 17α-methyl-B-norandrost-4-en-17β-ol-3-one, is a synthetic androstane steroid and a derivative of testosterone.[2] Specifically, it is the C17α methyl and B-nor analogue of testosterone and the B-nor analogue of methyltestosterone.[2] Other testosterone-derived steroidal antiandrogens include abiraterone acetate, BOMT, delanterone, dienogest, galeterone, metogest, mifepristone, oxendolone, rosterolone, topterone, trimethyltrienolone, and zanoterone, while progesterone-derived steroidal antiandrogens include examples like cyproterone and cyproterone acetate.[2]
History
Benorterone was developed in the late 1950s, was first reported to possess antiandrogenic activity in 1964, and was investigated in clinical trials in the mid-to-late 1960s.[2][1][6] It was the first known antiandrogen to be studied in humans.[1] The drug was found to be effective in the treatment of acne, seborrhea, and hirsutism in women.[3][15][16] In addition, unlike progestogenic antiandrogens such as cyproterone acetate, it seldom produced side effects in women and did not affect menstruation.[3] However, in males, benorterone was not effective for acne, and produced high rates of gynecomastia (in 12 out of 13 or 92% of young men treated with 75 to 300 mg/day benorterone).[17][18][19] Shortly following the observance of this side effect, it was withdrawn from clinical studies.[3][1] Subsequently, cyproterone acetate, which has a greatly reduced risk of gynecomastia by virtue of its concomitant progestogenic and antigonadotropic actions (which results in suppression of estrogen levels), was developed instead and was introduced for medical use in 1973.[20] In addition, spironolactone, a steroidal antimineralocorticoid that was introduced for medical use in 1959, was discovered to possess potent antiandrogenic activity in 1969, and became widely used clinically as an antiandrogen after its first use in an androgen-dependent condition in 1978.[21][22][23]
Society and culture
Generic names
Benorterone is the generic name of the drug and its INN and USAN.[2] It is also known by its developmental code names SKF-7690 and FC-612.[2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Advances in gynaecological endocrinology: proceedings of the Sixth Study Group of the Royal College of Obstetricians and Gynaecologists, 18th and 19th October, 1978. The College. 1979. p. 367. ISBN 978-0-87489-225-3. https://books.google.com/books?id=waMTAQAAMAAJ. "Limited clinical experience also exists with benorterone, the first anti-androgen tried in man, and with free cyproterone. In the late sixties benorterone was reported to give promising results in 93 androgenized women but was soon withdrawn from clinical trial, mainly because of the development of gynaecomastia in the male. As a big advantage compared with CPA, it was found to be effective not only orally but also topically. Free cyproterone, on the other hand, proved to be without clinical value for reasons that cannot be discussed here. Thus we are left with CPA as the only anti-androgen that is already on the market in several countries."
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 129–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA129.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 "Clinical Uses of Anti Androgens (other than for hypersexuality and sexual deviations)". Androgens II and Antiandrogens / Androgene II und Antiandrogene. Springer Science & Business Media. 27 November 2013. pp. 491–492, 516–517, 523. ISBN 978-3-642-80859-3. https://books.google.com/books?id=7JPsCAAAQBAJ&pg=PA491.
- ↑ 4.0 4.1 "Androgen antagonists in androgen target tissues". Pharmacology & Therapeutics 24 (3): 367–400. 1984. doi:10.1016/0163-7258(84)90010-X. PMID 6205409.
- ↑ "Screening for antiandrogenic activity of some 4,5-cyclo-A-homo-B-nor-and-androstane derivatives". Journal of Steroid Biochemistry 8 (9): 939–941. September 1977. doi:10.1016/0022-4731(77)90190-X. PMID 916678.
- ↑ 6.0 6.1 "An antiandrogen in acne and idiopathic hirsutism". The Journal of Investigative Dermatology 52 (4): 348–350. April 1969. doi:10.1038/jid.1969.58. PMID 4238084.
- ↑ Drug Management of Prostate Cancer. Springer Science & Business Media. 14 September 2010. pp. 71–. ISBN 978-1-60327-829-4. https://books.google.com/books?id=4KDrjeWA5-UC&pg=PA71.
- ↑ Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. 2011. pp. 80–. ISBN 978-0-7817-7968-5. https://books.google.com/books?id=Ll73ZsBKLkwC&pg=PA80.
- ↑ "The anti-androgenic activity of 17α-methyl-B-nortestosterone (SK&F 7690)". Steroids 3 (6): 687–698. 1964. doi:10.1016/0039-128X(64)90117-5. ISSN 0039-128X.
- ↑ "Hirsutism, virilism, polycystic ovarian disease, and the steroid-gonadotropin-feedback system: a career retrospective". American Journal of Physiology. Endocrinology and Metabolism 302 (1): E4–E18. January 2012. doi:10.1152/ajpendo.00488.2011. PMID 22028409.
- ↑ Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. 2001. pp. 1196–. ISBN 978-0-7817-1750-2. https://books.google.com/books?id=FVfzRvaucq8C&pg=PA1196.
- ↑ Anabolics. Molecular Nutrition Llc. 2011. pp. 533–. ISBN 978-0-9828280-1-4. https://books.google.com/books?id=afKLA-6wW0oC&pg=PT533.
- ↑ "Estrogens and Antiestrogens in the Male". Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. 6 December 2012. pp. 505–574 (543). ISBN 978-3-642-60107-1. https://books.google.com/books?id=wBvyCAAAQBAJ&pg=PA543.
- ↑ Fare LR, Kerwin JR, Kinney RW, "B-norestrogens", US patent 3377361, issued 9 April 1968, assigned to Smith Kline and French Laboratories Ltd.
- ↑ "Effect of an antiandrogen, 17-alpha-methyl-B-nortestosterone, on acne and hirsutism". The Journal of Clinical Endocrinology and Metabolism 26 (12): 1394–1398. December 1966. doi:10.1210/jcem-26-12-1394. PMID 4225258.
- ↑ Hair and Hair Diseases. Springer Science & Business Media. 1990. pp. 1195–. ISBN 978-3-642-74612-3. https://books.google.com/books?id=k7urBgAAQBAJ&pg=PT1195.
- ↑ "Antiandrogens: Clinical Aspects". Hair and Hair Diseases. Springer. 1990. pp. 827–886. doi:10.1007/978-3-642-74612-3_35. ISBN 978-3-642-74614-7.
- ↑ "Gynecomastia from a non-estrogenic anti-androgen". The Journal of Clinical Endocrinology and Metabolism 27 (9): 1348–1349. September 1967. doi:10.1210/jcem-27-9-1348. PMID 4227085.
- ↑ "Spontaneous pubertal breast growth in a castrated patient with the syndrome of testicular feminization". The Yale Journal of Biology and Medicine 44 (2): 225–229. October 1971. PMID 5123057.
- ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1182–. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA1182.
- ↑ "Anti-androgenic activity of spironolactone". Steroids 14 (4): 449–450. October 1969. doi:10.1016/S0039-128X(69)80007-3. PMID 5344274.
- ↑ "Spironolactone therapy for hirsutism in a hyperandrogenic woman". Annals of Internal Medicine 89 (5 Pt 1): 643–644. November 1978. doi:10.7326/0003-4819-89-5-643. PMID 717935.
- ↑ Glass' Office Gynecology. Lippincott Williams & Wilkins. 2014. pp. 47–. ISBN 978-1-60831-820-9. https://books.google.com/books?id=w2AGBAAAQBAJ&pg=PA47.
 |
