Chemistry:Bakuchiol
| |
| Names | |
|---|---|
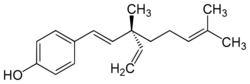
| Preferred IUPAC name
4-[(1E,3S)-3-Ethenyl-3,7-dimethylocta-1,6-dien-1-yl]phenol | |
| Other names
(+)-Bakuchiol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H24O | |
| Molar mass | 256.38 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bakuchiol is a meroterpenoid (a chemical compound having a partial terpenoid structure) in the class terpenophenol.[1]
It was first isolated in 1966 by Mehta et al. from Psoralea corylifolia seed and was called Bakuchiol based on the Sanskrit name of the plant, Bakuchi.[2] Bakuchiol is a meroterpene phenol abundant in[3] and mainly obtained from the seeds of the Psoralea corylifolia plant,[4][5][6] which is widely used in Indian Ayurveda[7] as well as in Traditional Chinese medicine[8] to treat a variety of diseases.[9] It has also been isolated from other plants, such as P. grandulosa,[10][11] P. drupaceae,[12] Ulmus davidiana,[13] Otholobium pubescens,[14] Piper longum[15] and Aerva sangulnolenta Blum.[16]
Even though the first complete synthesis of Bakuchiol was described in 1973,[17] its first commercial use in topical applications did not occur until 2007 when it was introduced to the market under the trade name Sytenol A by Sytheon Ltd.[18]
It has been reported to have anticancer activity in preclinical models, possibly due to its structural similarity with resveratrol.[19] One study in rats suggested that Bakuchiol and ethanol extracts of the Chinese medicinal plant Psoralea corylifolia could protect against bone loss.[20]
Bakuchiol possesses antioxidant,[21][22] anti-inflammatory,[23][24] and antibacterial[25] properties. Bakuchiol isolated from P. corylifolia has shown activity against numerous Gram-positive and Gram-negative oral pathogens. It was able to inhibit the growth of Streptococcus mutans under a range of sucrose concentrations, pH values and in the presence of organic acids in a temperature-dependent manner and also inhibited the growth of cells adhered to a glass surface.[26]
Despite having no structural resemblance to retinol,[27] Bakuchiol was found to have retinol functionality through retinol-like regulation of gene expression.[28][29] In 2018, a randomized, double-blind, 12-week clinical study with 44 volunteers demonstrated that Bakuchiol is comparable with retinol in its ability to improve photoaging (wrinkles, hyperpigmentation) but has a better skin tolerance.[30]
Bakuchiol has been found to possess antiandrogenic activity in prostate cancer cells, which inhibited cell proliferation.[31]
See also
References
- ↑ J. Elks; C. R. Ganellin (1990). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 120–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA120.
- ↑ Mehta, G.; Nayak, U.Ramdas; Dev, Sukh (January 1966). "Bakuchiol, a novel monoterpenoid". Tetrahedron Letters 7 (38): 4561–4567. doi:10.1016/s0040-4039(00)70078-5. ISSN 0040-4039.
- ↑ Chaudhuri, R. K.; Bojanowski, K. (2014). "Bakuchiol: a retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects" (in en). International Journal of Cosmetic Science 36 (3): 221–230. doi:10.1111/ics.12117. PMID 24471735. https://onlinelibrary.wiley.com/doi/10.1111/ics.12117.
- ↑ Banerji, A; Chintalwar, G (1983). "Biosynthesis of bakuchiol, a meroterpene from Psoralea corylifolia". Phytochemistry 22 (9): 1945–1947. doi:10.1016/0031-9422(83)80019-3. INIST:9311490
- ↑ Cho, Hyun; Jun, Jung-Yang; Song, Eun-Kyoung et al. (2001). "Bakuchiol: a hepatoprotective compound of Psoralea corylifolia on tacrine-induced cytotoxicity in Hep G2 cells". Planta Medica 67 (8): 750–751. doi:10.1055/s-2001-18347. PMID 11731920.
- ↑ Manohar, B.; Divakar, S.; Udaya Sankar, K (2009). "Amyloglucosidase Catalyzed Syntheses of Bakuchiol Glycosides in Supercritical Carbon Dioxide". Bulletin of the Korean Chemical Society 30 (8): 1760–1766. doi:10.5012/bkcs.2009.30.8.1760. INIST:22343814
- ↑ Does it make skin look younger?
- ↑ "Natural Ingredients - Organic Vegan Non-GMO - Grown Sustainably" (in en-US). https://theplantmother.com/ingredients/.
- ↑ Koul, B.; Taak, P.; Kumar, A.; Kumar, A.; Sanyal, I. (2019). "Genus Psoralea: A review of the traditional and modern uses, phytochemistry and pharmacology". Journal of Ethnopharmacology 232: 201–226. doi:10.1016/j.jep.2018.11.036. PMID 30521980.
- ↑ Labbé, Cecilia; Faini, Francesca; Coll, Joseph; Connolly, Joseph D. (July 1996). "Bakuchiol derivatives from the leaves of Psoralea glandulosa". Phytochemistry 42 (5): 1299–1303. doi:10.1016/0031-9422(96)00144-6. ISSN 0031-9422.
- ↑ Nadine Backhouse, C; Delporte, Carla L; Negrete, Rosa E; Erazo, Silvia; Zuñiga, Alexandra; Pinto, Alvaro; Cassels, Bruce K (November 2001). "Active constituents isolated from Psoralea glandulosa L. with antiinflammatory and antipyretic activities". Journal of Ethnopharmacology 78 (1): 27–31. doi:10.1016/s0378-8741(01)00309-9. ISSN 0378-8741. PMID 11585684.
- ↑ Lystvan, Kateryna; Belokurova, Valeria; Sheludko, Yuriy; Ingham, John L.; Prykhodko, Valeria; Kishchenko, Olena; Paton, Evgenija; Kuchuk, Mykola (2009-12-20). "Production of bakuchiol by in vitro systems of Psoralea drupacea Bge". Plant Cell, Tissue and Organ Culture 101 (1): 99–103. doi:10.1007/s11240-009-9657-0. ISSN 0167-6857.
- ↑ Choi, Sang Yoon; Lee, Sanghyun; Choi, Won-Hee; Lee, Yeonmi; Jo, Youn Ock; Ha, Tae-Youl (August 2010). "Isolation and Anti-Inflammatory Activity of Bakuchiol from Ulmus davidiana var. japonica". Journal of Medicinal Food 13 (4): 1019–1023. doi:10.1089/jmf.2009.1207. ISSN 1096-620X. PMID 20553183.
- ↑ Krenisky, Joann M.; Luo, Jian; Reed, Michael J. et al. (1999). "Isolation and Antihyperglycemic Activity of Bakuchiol from Otholobium pubescens (Fabaceae), a Peruvian Medicinal Plant Used for the Treatment of Diabetes.". Biological & Pharmaceutical Bulletin 22 (10): 1137–1140. doi:10.1248/bpb.22.1137. PMID 10549873. INIST:1198639
- ↑ Ohno, Osamu; Watabe, Taeko; Nakamura, Kazuhiko; Kawagoshi, Masaru; Uotsu, Nobuo; Chiba, Tomohiro; Yamada, Masayoshi; Yamaguchi, Kohji et al. (2010-07-23). "Inhibitory Effects of Bakuchiol, Bavachin, and Isobavachalcone Isolated from Piper longumon Melanin Production in B16 Mouse Melanoma Cells". Bioscience, Biotechnology, and Biochemistry 74 (7): 1504–1506. doi:10.1271/bbb.100221. ISSN 0916-8451. PMID 20622433.
- ↑ "Aerva Sanguinolenta (L.) Blume, Extract 116425-35-5", Sax's Dangerous Properties of Industrial Materials, John Wiley & Sons, Inc., 2012-10-15, doi:10.1002/0471701343.sdp26534, ISBN 978-0-471-70134-7
- ↑ Damodaran, N.P.; Dev, Sukh (January 1973). "Meroterpenoids—III". Tetrahedron 29 (9): 1209–1213. doi:10.1016/0040-4020(73)80103-6. ISSN 0040-4020.
- ↑ Chaudhuri, Ratan (2015-09-18), Sivamani, Raja; Jagdeo, Jared; Elsner, Peter et al., eds., "Bakuchiol: A Retinol-Like Functional Compound, Modulating Multiple Retinol and Non-Retinol Targets" (in en), Cosmeceuticals and Active Cosmetics, Third Edition (CRC Press): pp. 1–18, doi:10.1201/b18895-2, ISBN 978-1-4822-1416-1, http://www.crcnetbase.com/doi/10.1201/b18895-2, retrieved 2019-08-02[yes|permanent dead link|dead link}}]
- ↑ Chen, Zhe; Jin, Ke; Gao, Lingyan et al. (2010). "Anti-tumor effects of bakuchiol, an analogue of resveratrol, on human lung adenocarcinoma A549 cell line". European Journal of Pharmacology 643 (2–3): 170–9. doi:10.1016/j.ejphar.2010.06.025. PMID 20599920.
- ↑ Lim, Sun-Hye; Ha, Tae-Youl; Kim, Sung-Ran et al. (2008). "Ethanol extract of Psoralea corylifolia L. and its main constituent, bakuchiol, reduce bone loss in ovariectomised Sprague–Dawley rats". British Journal of Nutrition 101 (7): 1031–1039. doi:10.1017/S0007114508066750. PMID 18801207.
- ↑ Adhikari, S.; Joshi, R.; Patro, B. S.; Ghanty, T. K.; Chintalwar, G. J.; Sharma, A.; Chattopadhyay, S.; Mukherjee, T. (September 2003). "Antioxidant Activity of Bakuchiol: Experimental Evidences and Theoretical Treatments on the Possible Involvement of the Terpenoid Chain". Chemical Research in Toxicology 16 (9): 1062–1069. doi:10.1021/tx034082r. ISSN 0893-228X. PMID 12971793.
- ↑ Haraguchi, Hiroyuki; Inoue, Junji; Tamura, Yukiyoshi; Mizutani, Kenji (2002). "Antioxidative components of Psoralea corylifolia (Leguminosae)". Phytotherapy Research 16 (6): 539–544. doi:10.1002/ptr.972. ISSN 0951-418X. PMID 12237811.
- ↑ Ferrándiz, María Luisa; Gil, Blanca; Sanz, María Jesús; Ubeda, Amalia; Erazo, Silvia; González, Ernesto; Negrete, Rosa; Pacheco, Sergio et al. (September 1996). "Effect of Bakuchiol on Leukocyte Functions and Some Inflammatory Responses in Mice". Journal of Pharmacy and Pharmacology 48 (9): 975–980. doi:10.1111/j.2042-7158.1996.tb06016.x. ISSN 0022-3573. PMID 8910867.
- ↑ Nadine Backhouse, C; Delporte, Carla L; Negrete, Rosa E; Erazo, Silvia; Zuñiga, Alexandra; Pinto, Alvaro; Cassels, Bruce K (November 2001). "Active constituents isolated from Psoralea glandulosa L. with antiinflammatory and antipyretic activities". Journal of Ethnopharmacology 78 (1): 27–31. doi:10.1016/s0378-8741(01)00309-9. ISSN 0378-8741. PMID 11585684.
- ↑ Katsura, H.; Tsukiyama, R.-I.; Suzuki, A.; Kobayashi, M. (2001-11-01). "In Vitro Antimicrobial Activities of Bakuchiol against Oral Microorganisms". Antimicrobial Agents and Chemotherapy 45 (11): 3009–3013. doi:10.1128/aac.45.11.3009-3013.2001. ISSN 0066-4804. PMID 11600349.
- ↑ Parimala Devi B, Ramasubramaniaraj R (2009). "Dental Caries and Medicinal Plants – An Overview". Journal of Pharmacy Research 2 (11): 1669–1675. http://jprsolutions.info/article_detail.php?article_id=632.
- ↑ Dhaliwal, S.; Rybak, I.; Ellis, S.R.; Notay, M.; Trivedi, M.; Burney, W.; Vaughn, A.R.; Nguyen, M. et al. (2019). "Prospective, randomized, double‐blind assessment of topical bakuchiol and retinol for facial photoageing" (in en). British Journal of Dermatology 180 (2): 289–296. doi:10.1111/bjd.16918. ISSN 0007-0963. PMID 29947134. https://onlinelibrary.wiley.com/doi/10.1111/bjd.16918.
- ↑ Chaudhuri RK, Bojanowski K (2014). "Bakuchiol: a retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects". International Journal of Cosmetic Science 36 (3): 221–230. doi:10.1111/ics.12117. PMID 24471735.
- ↑ Chaudhuri, R. K.; Bojanowski, K. (2014). "Bakuchiol: a retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects" (in en). International Journal of Cosmetic Science 36 (3): 221–230. doi:10.1111/ics.12117. PMID 24471735. https://onlinelibrary.wiley.com/doi/10.1111/ics.12117.
- ↑ Dhaliwal, S.; Rybak, I.; Ellis, S.R.; Notay, M.; Trivedi, M.; Burney, W.; Vaughn, A.R.; Nguyen, M. et al. (February 2019). "Prospective, randomized, double‐blind assessment of topical bakuchiol and retinol for facial photoageing". British Journal of Dermatology 180 (2): 289–296. doi:10.1111/bjd.16918. ISSN 0007-0963. PMID 29947134.
- ↑ Miao L. et al. (2013). "Bakuchiol inhibits the androgen induced-proliferation of prostate cancer cell line LNCaP through suppression of AR transcription activity". Tianjin Journal of Traditional Chinese Medicine 30 (5): 291–293. doi:10.11656/j.issn.1672-1519.2013.05.13. original title: 补骨脂酚拮抗AR转录活性抑制雄激素诱导的前列腺癌细胞LNCaP的增殖
External links
 |
