Chemistry:Proxalutamide
 | |
| Clinical data | |
|---|---|
| Other names | Pruxelutamide; GT-0918 |
| Routes of administration | By mouth |
| Drug class | Nonsteroidal antiandrogen |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
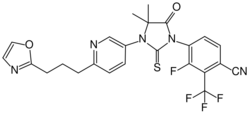
| Formula | C24H19F4N5O2S |
| Molar mass | 517.50 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Proxalutamide (developmental code name GT-0918) is a nonsteroidal antiandrogen (NSAA) – specifically, a selective high-affinity silent antagonist of the androgen receptor (AR) – which is under development by Suzhou Kintor Pharmaceuticals, inc., a subsidiary of Kintor Pharmaceutical Limited, for the potential treatment of COVID-19, prostate cancer, and breast cancer.[1][2][3][4][5] It was approved in Paraguay for the treatment of COVID-19 in July 2021, but has not been approved at this time in other countries.[1]
Research
Prostate cancer
Proxalutamide is in phase III studies for mCRPC as monotherapy and in combination with abiraterone. In the United States, it is in phase II study as monotherapy for mCRPC.[6][7]
Other indications
Proxalutamide is in phase Ic clinical trial in China.[1][8]
See also
References
- ↑ 1.0 1.1 1.2 "Proxalutamide - Suzhou Kintor Pharmaceuticals". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800046361.
- ↑ "Proxalutamide (GT0918), a potent androgen receptor pathway inhibitor". Cancer Research 74 (19 Supplement): 614. 2014. doi:10.1158/1538-7445.AM2014-614. ISSN 0008-5472.
- ↑ "Discovery of potent androgen receptor antagonists for treating CRPC and AR+ breast cancer". Cancer Research 73 (8 Supplement): 2460. 2014. doi:10.1158/1538-7445.AM2013-2460. ISSN 0008-5472.
- ↑ Applied Biology, Inc. (2020-12-29). Anti-Androgen Treatment for COVID-19. https://clinicaltrials.gov/ct2/show/NCT04446429.
- ↑ "EQS-News: Kintor's Proxalutamide (GT0918) COVID-19 Clinical Trial Shows Positive Re-sults in Treatment of Male and Female" (in en). Bloomberg.com. 2021-01-11. https://www.bloomberg.com/press-releases/2021-01-11/eqs-news-kintor-s-proxalutamide-gt0918-covid-19-clinical-trial-shows-positive-re-sults-in-treatment-of-male-and-female.
- ↑ "Product Pipeline". https://en.kintor.com.cn/intro/30.html.
- ↑ Suzhou Kintor Pharmaceutical (2020-03-13). "An Extended/Phase 2, Multi-Center, Randomized, Open-Label Study to Evaluate the Safety and Tolerability of GT0918 in Subjects With Metastatic Castrate Resistant Prostate Cancer (mCRPC) Who Failed Either Abiraterone or Enzalutamide". Clinical Trials (US). https://clinicaltrials.gov/ct2/show/NCT03899467.
- ↑ Safety, Tolerability, and Pharmacokinetics of Proxalutamide Therapy in Women With Metastatic Breast Cancer. 24 September 2019. https://clinicaltrials.gov/ct2/show/NCT04103853. Retrieved 2021-01-29.
 |
