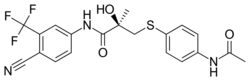
Chemistry:Acetothiolutamide
 | |
| Clinical data | |
|---|---|
| Other names | Thioacetolutamide |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C20H18F3N3O3S |
| Molar mass | 437.44 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Acetothiolutamide is a selective androgen receptor modulator (SARM) derived from the nonsteroidal antiandrogen bicalutamide that was described in 2002 and was one of the first SARMs to be discovered and developed.[1][2][3][4] It is a high-affinity, selective ligand of the androgen receptor (AR) (Ki = 2.1–4.9 nM), where it acts as a full agonist in vitro, and has in vitro potency comparable to that of testosterone.[2][4][5] However, in vivo, acetothiolutamide displayed overall negligible androgenic effects, though significant (albeit very low) anabolic effects were observed at high doses.[2] In addition, notable antiandrogen effects were observed in castrated male rats treated with testosterone propionate.[2] The discrepancy between the in vitro and in vivo actions of acetothiolutamide was determined to be related to rapid plasma clearance and extensive hepatic metabolism into a variety of metabolites with differing pharmacological activity, including AR partial agonism and antagonism.[2][4][6] In accordance with its poor metabolic stability, acetothiolutamide is not orally bioavailable, and shows activity only via injected routes such as subcutaneous and intravenous.[2]
See also
References
- ↑ "Discovery of nonsteroidal androgens". Biochemical and Biophysical Research Communications 244 (1): 1–4. March 1998. doi:10.1006/bbrc.1998.8209. PMID 9514878.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Pharmacology, pharmacokinetics, and metabolism of acetothiolutamide, a novel nonsteroidal agonist for the androgen receptor". The Journal of Pharmacology and Experimental Therapeutics 304 (3): 1323–33. March 2003. doi:10.1124/jpet.102.040832. PMID 12604713.
- ↑ Kearbey, Jeffrey Dale (2004), Preclinical Pharmacokinetics and Skeletal Pharmacology of a Selective Androgen Receptor Modulator, https://etd.ohiolink.edu/!etd.send_file?accession=osu1085168433&disposition=attachment
- ↑ 4.0 4.1 4.2 "In vivo metabolism and final disposition of a novel nonsteroidal androgen in rats and dogs". Drug Metabolism and Disposition 34 (10): 1713–21. October 2006. doi:10.1124/dmd.106.009985. PMID 16815963.
- ↑ "The para substituent of S-3-(phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamides is a major structural determinant of in vivo disposition and activity of selective androgen receptor modulators". The Journal of Pharmacology and Experimental Therapeutics 315 (1): 230–9. October 2005. doi:10.1124/jpet.105.088344. PMID 15987833.
- ↑ "Pharmacodynamics of selective androgen receptor modulators". The Journal of Pharmacology and Experimental Therapeutics 304 (3): 1334–40. March 2003. doi:10.1124/jpet.102.040840. PMID 12604714.
 |
