Chemistry:Metenolone
 | |
| Clinical data | |
|---|---|
| Trade names | Primobolan, Nibal (as metenolone acetate); Primobolan Depot, Nibal Injection (as metenolone enanthate) |
| Other names | Methenolone; Methylandrostenolone; 1-Methyl-δ1-4,5α-dihydrotestosterone; 1-Methyl-δ1-DHT; 1-Methyl-5α-androst-1-en-17β-ol-3-one |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth (as metenolone acetate), intramuscular injection (as metenolone enanthate) |
| Drug class | Androgen; Anabolic steroid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H30O2 |
| Molar mass | 302.458 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Metenolone, or methenolone, is an androgen and anabolic steroid (AAS) which is used in the form of esters such as metenolone acetate (brand name Primobolan, Nibal) and metenolone enanthate (brand name Primobolan Depot, Nibal Injection).[1][2][3][4][5] Metenolone esters are used mainly in the treatment of anemia due to bone marrow failure.[6] Metenolone acetate is taken by mouth, while metenolone enanthate is given by injection into muscle.[5]
Side effects of metenolone esters include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[5] Metenolone esters are synthetic androgens and anabolic steroids and hence are agonists of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[5][7] They have moderate anabolic effects and weak androgenic effects, as well as no estrogenic effects or risk of liver damage.[5][7] Metenolone esters are androgen esters and prodrugs of metenolone in the body.[5]
Metenolone esters were introduced for medical use in the early 1960s.[5] In addition to their medical use, metenolone esters are used to improve physique and performance.[5] The drugs are controlled substances in many countries and so non-medical use is generally illicit.[5] They have mostly been discontinued for medical use and have limited availability.[4][5]
Medical uses
Metenolone, as its esters, is used almost exclusively in the treatment of anemia due to bone marrow failure.[6] It has also been used to treat wasting syndromes due to major surgery, infection, long-term corticosteroid therapy, malnutrition, or other causes.[5] It has also been used to treat osteoporosis and sarcopenia, to inhibit the natural loss of muscle mass with aging, and to promote weight gain in underweight premature infants and children.[5]
Side effects
Side effects of metenolone and its esters include virilization among others.[5]
Pharmacology
Pharmacodynamics
| Medication | Ratioa |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: a = Ratio of androgenic to anabolic activity. Sources: See template. | |
Due to its double bond between the C1 and C2 positions, metenolone is resistant to metabolism by 3α-hydroxysteroid dehydrogenase (3α-HSD).[5] As such, unlike DHT and the closely related DHT derivatives mestanolone (17α-methyl-DHT) and mesterolone (1α-methyl-DHT), metenolone has considerable anabolic effects.[5]
Pharmacokinetics
Metenolone has very low affinity for human serum sex hormone-binding globulin (SHBG), about 16% of that of testosterone and 3% of that of DHT.[8]
Chemistry
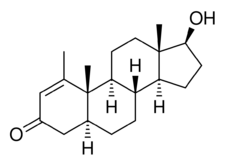
Metenolone, also known as 1-methyl-4,5α-dihydro-δ1-testosterone (1-methyl-δ1-DHT) or as 1-methyl-5α-androst-1-en-17β-ol-3-one, is a synthetic androstane steroid and derivative of dihydrotestosterone (DHT).[1][2][5] A closely related AAS is mesterolone (1α-methyl-DHT).[1][2][5]
Society and culture
Generic names
Metenolone is the generic name of the drug and its INN, while methenolone is its BAN.[1][2][3][4] It has also been referred to as methylandrostenolone.[2][4] This synonym should not be confused with methandrostenolone, which is another name for a different AAS known as metandienone.[9]
Doping in sports
Metenolone and its esters are banned from use in sports governed by the World Anti-Doping Agency.[10] The NBA and NBPA also banned the use of metenolone and its esters under the Anti-Drug Program. There are known cases of doping in sports with metenolone esters by professional athletes.
References
- ↑ 1.0 1.1 1.2 1.3 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 784–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA784.
- ↑ 2.0 2.1 2.2 2.3 2.4 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 659–660. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA660.
- ↑ 3.0 3.1 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 178–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA178.
- ↑ 4.0 4.1 4.2 4.3 "List of Androgens and anabolic steroids". Drugs.com. https://www.drugs.com/international/metenolone.html.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 625–,633–. ISBN 978-0-9828280-1-4. https://books.google.com/books?id=afKLA-6wW0oC&pg=PT625.
- ↑ 6.0 6.1 "Androgen Physiology, Pharmacology, and Abuse". Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. 25 February 2015. pp. 2388–. ISBN 978-0-323-32195-2. https://books.google.com/books?id=xmLeBgAAQBAJ&pg=PA2388.
- ↑ 7.0 7.1 "Pharmacology of anabolic steroids". British Journal of Pharmacology 154 (3): 502–521. June 2008. doi:10.1038/bjp.2008.165. PMID 18500378.
- ↑ "Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin". Endocrinology 114 (6): 2100–2106. June 1984. doi:10.1210/endo-114-6-2100. PMID 6539197.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. p. 660. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PT660.
- ↑ "The World Anti-Doping Code: The 2012 Prohibited List". World Anti-Doping Agency. http://www.wada-ama.org/Documents/World_Anti-Doping_Program/WADP-Prohibited-list/2012/WADA_Prohibited_List_2012_EN.pdf.
External links
- Primobolan (methenolone acetate) - William Llewellyn's Anabolic.org
- Primobolan Depot (methenolone enanthate) - William Llewellyn's Anabolic.org
{{Navbox
| name = Androgens and antiandrogens | title = Androgens and antiandrogens | state = collapsed | listclass = hlist | groupstyle = text-align:center;
| group1 = Androgens
(incl. AAS)
| list1 =
| group2 = Antiandrogens | list2 = {{Navbox|child | groupstyle = text-align:center; | groupwidth = 9em;
| group1 = AR antagonists | list1 =
- Steroidal: Abiraterone acetate
- Canrenone
- Chlormadinone acetate
- Cyproterone acetate
- Delmadinone acetate
- Dienogest
- Drospirenone
- Medrogestone
- Megestrol acetate
- Nomegestrol acetate
- Osaterone acetate
- Oxendolone
- Potassium canrenoate
- Spironolactone
- Nonsteroidal: Apalutamide
- Bicalutamide
- Cimetidine
- Darolutamide
- Enzalutamide
- Flutamide
- Ketoconazole
- Nilutamide
- Seviteronel†
- Topilutamide (fluridil)
| group2 = Steroidogenesis| list2 =
inhibitors
| 5α-Reductase | |
|---|---|
| Others |
| group3 = Antigonadotropins | list3 =
- D2 receptor antagonists (prolactin releasers) (e.g., domperidone, metoclopramide, risperidone, haloperidol, chlorpromazine, sulpiride)
- Estrogens (e.g., bifluranol, [[diethylstilbestrol, estradiol, estradiol esters, ethinylestradiol, ethinylestradiol sulfonate, paroxypropione)
- GnRH agonists (e.g., leuprorelin)
- GnRH antagonists (e.g., cetrorelix)
- Progestogens (incl., chlormadinone acetate, [[cyproterone acetate, hydroxyprogesterone caproate, gestonorone caproate, [[Chemistry:Medroxyprogesterone medroxyprogesterone acetate, Chemistry:Megestrol acetate|megestrol acetate]])
| group4 = Others | list4 =
- Androstenedione immunogens: Androvax (androstenedione albumin)
- Ovandrotone albumin (Fecundin)
}}
| liststyle = background:#DDDDFF;| list3 =
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
- See also
- Androgen receptor modulators
- Estrogens and antiestrogens
- Progestogens and antiprogestogens
- List of androgens/anabolic steroids
}}
 |
