Chemistry:Alternariol
From HandWiki
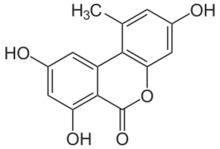
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,7,9-Trihydroxy-1-methyl-6H-dibenzo[b,d]pyran-6-one | |
| Other names
3,7,9-Trihydroxy-1-methyl-6H-benzo[c]chromen-6-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H10O5 | |
| Molar mass | 258.229 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Alternariol is a toxic metabolite of Alternaria fungi.[1] It is an important contaminant in cereals and fruits.[2] Alternariol exhibits antifungal and phytotoxic activity. It is reported to inhibit cholinesterase enzymes.[3] It is also a mycoestrogen.
Alternariol is reported to be a full androgen agonist in an in vitro assay.[4]
References
- ↑ "Evaluation of alternariol and alternariol methyl ether for mutagenic activity in Salmonella typhimurium". Appl. Environ. Microbiol. 60 (10): 3901–2. 1 October 1994. doi:10.1128/AEM.60.10.3901-3902.1994. PMID 7986060. Bibcode: 1994ApEnM..60.3901D.
- ↑ "Mutagenicity of the mycotoxin alternariol in cultured mammalian cells". Toxicol. Lett. 164 (3): 221–30. 2006. doi:10.1016/j.toxlet.2006.01.001. PMID 16464542.
- ↑ Alternariol product page from Fermentek
- ↑ Stypuła-Trębas, Sylwia; Minta, Maria; Radko, Lidia; Jedziniak, Piotr; Posyniak, Andrzej (2017). "Nonsteroidal mycotoxin alternariol is a full androgen agonist in the yeast reporter androgen bioassay". Environmental Toxicology and Pharmacology 55: 208–211. doi:10.1016/j.etap.2017.08.036. ISSN 1382-6689. PMID 28910742.
 |
