Chemistry:Dicirenone
From HandWiki
Short description: Chemical compound
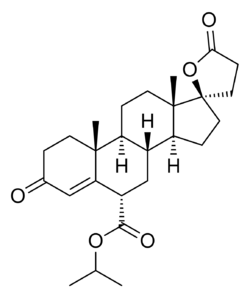 | |
| Clinical data | |
|---|---|
| Other names | SC-26304; 7α-Carboxyisopropylspirolactone; 17α-Hydroxy-3-oxopregn-4-ene-7α,21-dicarboxylic acid γ-lactone 1-isopropyl ester |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H36O5 |
| Molar mass | 428.569 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Dicirenone (INN, USAN; developmental code name SC-26304; also known as 7α-carboxyisopropylspirolactone) is a synthetic, steroidal antimineralocorticoid of the spirolactone group which was developed as a diuretic and antihypertensive agent but was never marketed.[1][2] It was synthesized and assayed in 1974.[1] Similarly to other spirolactones like spironolactone, dicirenone also possesses antiandrogen activity, albeit with relatively reduced affinity.[3]
References
- ↑ 1.0 1.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 387–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA387.
- ↑ Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 97–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA97.
- ↑ "Relative inhibitory potency of five mineralocorticoid antagonists on aldosterone biosynthesis in vitro". Biochemical Pharmacology 34 (2): 189–194. January 1985. doi:10.1016/0006-2952(85)90123-6. PMID 2981534.
 |
