Chemistry:Mexrenone
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
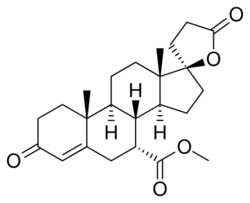
| Other names | ZK-32055; SC-25152; 7α-(Methoxycarbonyl)canrenone; 17β-Hydroxy-3-oxo-17α-pregn-4-ene-7α,21-dicarboxylic acid γ-lactone methyl ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C24H32O5 |
| Molar mass | 400.515 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Mexrenone (code names ZK-32055, SC-25152) is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone that was never marketed.[1][2] It is the lactonic form of mexrenoic acid (mexrenoate), and mexrenoate potassium (SC-26714), the potassium salt of mexrenoic acid, also exists.[3] In addition to the mineralocorticoid receptor, mexrenone also binds to the glucocorticoid, androgen, and progesterone receptors.[4] Relative to spironolactone, it has markedly reduced antiandrogen activity (approximately one-tenth of the antimineralocorticoid dosage equivalent antiandrogen activity of spironolactone).[2] Eplerenone is the 9-11α-epoxy analogue of mexrenone.[5]
See also
References
- ↑ "SC 25152: A potent mineralocorticoid antagonist with reduced affinity for the 5 alpha-dihydrotestosterone receptor of human and rat prostate". The Journal of Clinical Endocrinology and Metabolism 47 (1): 171–175. July 1978. doi:10.1210/jcem-47-1-171. PMID 263288.
- ↑ 2.0 2.1 "SC 25152: a potent mineralocorticoid antagonist with decreased antiandrogenic activity relative to spironolactone". The Journal of Pharmacology and Experimental Therapeutics 209 (1): 144–146. April 1979. PMID 430374.
- ↑ "Mexrenoate potassium: a steroidal aldosterone antagonist and antihypertensive". The Journal of Pharmacology and Experimental Therapeutics 201 (3): 762–768. June 1977. PMID 864608.
- ↑ Pharmaceutical Chemistry of Antihypertensive Agents. CRC Press. 19 December 1990. pp. 87–. ISBN 978-0-8493-4724-5. https://books.google.com/books?id=LMl4Sd38q4kC&pg=PA87.
- ↑ "The 45-year story of the development of an anti-aldosterone more specific than spironolactone". Molecular and Cellular Endocrinology 217 (1–2): 45–52. March 2004. doi:10.1016/j.mce.2003.10.008. PMID 15134800.
 |
