Chemistry:Β-Carboline
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
9H-Pyrido[3,4-b]indole | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 128414 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | norharman |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H8N2 | |
| Molar mass | 168.20 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
β-Carboline (9H-pyrido[3,4-b]indole) represents the basic chemical structure for more than one hundred alkaloids and synthetic compounds. The effects of these substances depend on their respective substituent. Natural β-carbolines primarily influence brain functions but can also exhibit antioxidant[1] effects. Synthetically designed β-carboline derivatives have recently been shown to have neuroprotective,[2] cognitive enhancing and anti-cancer properties.[3]
Pharmacology
The pharmacological effects of specific β-carbolines are dependent on their substituents. For example, the natural β-carboline harmine has substituents on position 7 and 1. Thereby, it acts as a selective inhibitor of the DYRK1A protein kinase, a molecule necessary for neurodevelopment.[4][5] It also exhibits various antidepressant-like effects in rats by interacting with serotonin receptor 2A.[6][7] Furthermore, it increases levels of the brain-derived neurotrophic factor (BDNF) in rat hippocampus.[7][8] A decreased BDNF level has been associated with major depression in humans. The antidepressant effect of harmine might also be due to its function as a MAO-A inhibitor by reducing the breakdown of serotonin and noradrenaline.[8][9]
A synthetic derivative, 9-methyl-β-carboline, has shown neuroprotective effects including increased expression of neurotrophic factors and enhanced respiratory chain activity.[10][11] This derivative has also been shown to enhance cognitive function,[12] increase dopaminergic neuron count and facilitate synaptic and dendritic proliferation.[13][14] It also exhibited therapeutic effects in animal models for Parkinson's disease and other neurodegenerative processes.[11]
However, β-carbolines with substituents in position 3 reduce the effect of benzodiazepine on GABA-A receptors and can therefore have convulsive, anxiogenic and memory enhancing effects.[15] Moreover, 3-hydroxymethyl-beta-carboline blocks the sleep-promoting effect of flurazepam in rodents and - by itself - can decrease sleep in a dose-dependent manner.[16] Another derivative, methyl-β-carboline-3-carboxylate, stimulates learning and memory at low doses but can promote anxiety and convulsions at high doses.[15] With modification in position 9 similar positive effects have been observed for learning and memory without promotion of anxiety or convulsion.[12]
β-carboline derivatives also enhance the production of the antibiotic reveromycin A in soil dwelling "Streptomyces" species.[17][18] Specifically, expression of biosynthetic genes is facilitated by binding of the β-carboline to a large ATP-binding regulator of the LuxR family.
Also Lactobacillus spp. secretes a β-carboline (1-acetyl-β-carboline) preventing the pathogenic fungus Candida albicans to change to a more virulent growth form (yeast-to-filament transition). Thereby, β-carboline reverses imbalances in the microbiome composition causing pathologies ranging from vaginal candidiasis to fungal sepsis.[19]
Since β-carbolines also interact with various cancer-related molecules such as DNA, enzymes (GPX4, kinases, etc.) and proteins (ABCG2/BRCP1, etc.), they are also discussed as potential anticancer agents.[3]
Explorative human studies for the medical use of β-carbolines
The extract of the liana Banisteriopsis caapi has been used by the tribes of the Amazon as an entheogen and was described as a hallucinogen in the middle of the 19th century.[20] In early 20th century, European pharmacists identified harmine as the active substance.[21] This discovery stimulated the interest to further investigate its potential as a medicine. For example, Louis Lewin, a prominent pharmacologist, demonstrated a dramatic benefit in neurological impairments after injections of B. caapi in patients with postencephalitic Parkinsonism.[20] By 1930, it was generally agreed that hypokinesia, drooling, mood, and sometimes rigidity improved by treatment with harmine. Altogether, 25 studies had been published in the 1920s and 1930s about patients with Parkinson's disease and postencephalitic Parkinsonism. The pharmacological effects of harmine have been attributed mainly to its central monoamine oxidase (MAO) inhibitory properties. In-vivo and rodent studies have shown that extracts of Banisteriopsis caapi and also Peganum harmala lead to striatal dopamine release.[22][23][24] Furthermore, harmine supports the survival of dopaminergic neurons in MPTP-treated mice.[25] Since harmine also antagonizes N-methyl-d-aspartate (NMDA) receptors,[26] some researchers speculatively attributed the rapid improvement in patients with Parkinson's disease to these antiglutamatergic effects.[20] However, the advent of synthetic anticholinergic drugs at that time led to the total abandonment of harmine.[20]
Structure
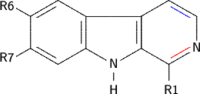
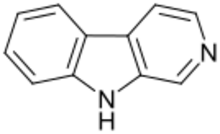
β-Carbolines belong to the group of indole alkaloids and consist of a pyridine ring that is fused to an indole skeleton.[27] The structure of β-carboline is similar to that of tryptamine, with the ethylamine chain re-connected to the indole ring via an extra carbon atom, to produce a three-ringed structure. The biosynthesis of β-carbolines is believed to follow this route from analogous tryptamines.[28] Different levels of saturation are possible in the third ring which is indicated here in the structural formula by coloring the optionally double bonds red and blue:
Examples of β-carbolines
Some of the more important β-carbolines are tabulated by structure below. Their structures may contain the aforementioned bonds marked by red or blue.
| Short Name | R1 | R6 | R7 | R9 | Structure |
|---|---|---|---|---|---|
| β-Carboline | H | H | H | H | 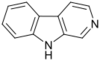
|
| Pinoline | H | OCH3 | H | H | 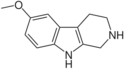
|
| Harmane | CH3 | H | H | H | 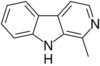
|
| Harmine | CH3 | H | OCH3 | H | 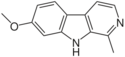
|
| Harmaline | CH3 | H | OCH3 | H | 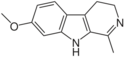
|
| Harmalol | CH3 | H | OH | H | 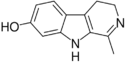
|
| Tetrahydroharmine | CH3 | H | OCH3 | H | 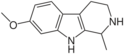
|
| 9-Methyl-β-carboline | H | H | H | CH3 | 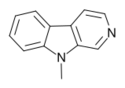
|
| 3-Carboxy-Tetrahydrononharman | H / CH3 / COOH | H | H | H | 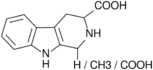
|
Natural occurrence

β-Carboline alkaloids are widespread in prokaryotes, plants and animals. Some β-carbolines, notably tetrahydro-β-carbolines, may be formed naturally in plants and the human body with tryptophan, serotonin and tryptamine as precursors.
- Altogether, eight plant families are known to express 64 different kinds of β-carboline alkaloids. For example, the β-carbolines harmine, harmaline, and tetrahydroharmine are components of the liana Banisteriopsis caapi and play a pivotal role in the pharmacology of the indigenous psychedelic drug ayahuasca. Moreover, the seeds of Peganum harmala (Syrian Rue) contain between 0.16%[29] and 5.9%[30] β-carboline alkaloids (by dry weight).
- A specific group of β-carboline derivatives, termed eudistomins, were extracted from ascidians (marine tunicates of the family Ascidiacea) such as Ritterella sigillinoides,[31] Lissoclinum fragile [32] or Pseudodistoma aureum.[33]
- Nostocarboline was isolated from freshwater cyanobacterium.[34]
- The fully aromatic β-carbolines also occur in many foodstuffs, however in lower concentrations. The highest amounts have been detected in brewed coffee, raisins, well done fish and meats.[35] Smoking is another source of fully aromatic β-carbolines with levels up to thousands of µg per smoker each day.[36]
- β-Carbolines have also been found in the cuticle of scorpions, causing their skin to fluoresce upon exposed to ultraviolet light at certain wavelengths (e.g. blacklight).[37]
See also
- Gamma-carboline
- Harmala alkaloid
- Oxopropaline[38]
- Tryptamine
References
- ↑ "Antioxidant activity of beta-carboline derivatives". Acta Poloniae Pharmaceutica 68 (2): 185–189. March 2011. PMID 21485291.
- ↑ "Good guys from a shady family". Journal of Neurochemistry 121 (6): 841–842. June 2012. doi:10.1111/j.1471-4159.2012.07708.x. PMID 22372749.
- ↑ 3.0 3.1 "β-Carbolines as potential anticancer agents". European Journal of Medicinal Chemistry 216: 113321. April 2021. doi:10.1016/j.ejmech.2021.113321. PMID 33684825.
- ↑ "Harmine treatment enhances short-term memory in old rats: Dissociation of cognition and the ability to perform the procedural requirements of maze testing". Physiology & Behavior 138: 260–265. January 2015. doi:10.1016/j.physbeh.2014.09.001. PMID 25250831.
- ↑ "Activation, regulation, and inhibition of DYRK1A". The FEBS Journal 278 (2): 246–256. January 2011. doi:10.1111/j.1742-4658.2010.07956.x. PMID 21126318.
- ↑ "Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors". Drug and Alcohol Dependence 60 (2): 121–132. August 2000. doi:10.1016/s0376-8716(99)00148-9. PMID 10940539.
- ↑ 7.0 7.1 "Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus". Progress in Neuro-Psychopharmacology & Biological Psychiatry. Bed nucleus of the stria terminalis: anatomy, physiology, functions 33 (8): 1425–1430. November 2009. doi:10.1016/j.pnpbp.2009.07.021. PMID 19632287.
- ↑ 8.0 8.1 "Chronic administration of harmine elicits antidepressant-like effects and increases BDNF levels in rat hippocampus". Journal of Neural Transmission 117 (10): 1131–1137. October 2010. doi:10.1007/s00702-010-0451-2. PMID 20686906.
- ↑ "Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today". Current Pharmaceutical Design 15 (14): 1563–1586. 2009-05-01. doi:10.2174/138161209788168001. PMID 19442174.
- ↑ (in en-gb) Isoquinolines And Beta-Carbolines As Neurotoxins And Neuroprotectants. 2012. doi:10.1007/978-1-4614-1542-8. ISBN 978-1-4614-1541-1.
- ↑ 11.0 11.1 "9-Methyl-beta-carboline has restorative effects in an animal model of Parkinson's disease". Pharmacological Reports 62 (1): 35–53. January 2010. doi:10.1016/s1734-1140(10)70241-3. PMID 20360614.
- ↑ 12.0 12.1 "9-Methyl-β-carboline-induced cognitive enhancement is associated with elevated hippocampal dopamine levels and dendritic and synaptic proliferation". Journal of Neurochemistry 121 (6): 924–931. June 2012. doi:10.1111/j.1471-4159.2012.07713.x. PMID 22380576.
- ↑ "9-Methyl-beta-carboline up-regulates the appearance of differentiated dopaminergic neurones in primary mesencephalic culture". Neurochemistry International 52 (4–5): 688–700. March 2008. doi:10.1016/j.neuint.2007.08.018. PMID 17913302.
- ↑ "Stimulation, protection and regeneration of dopaminergic neurons by 9-methyl-β-carboline: a new anti-Parkinson drug?". Expert Review of Neurotherapeutics 11 (6): 845–860. June 2011. doi:10.1586/ern.11.1. PMID 21651332.
- ↑ 15.0 15.1 "From the behavioral pharmacology of beta-carbolines to seizures, anxiety, and memory". TheScientificWorldJournal 7: 204–223. February 2007. doi:10.1100/tsw.2007.48. PMID 17334612.
- ↑ "A benzodiazepine receptor antagonist decreases sleep and reverses the hypnotic actions of flurazepam". Science 219 (4583): 414–416. January 1983. doi:10.1126/science.6294835. PMID 6294835. Bibcode: 1983Sci...219..414M.
- ↑ "β-carboline biomediators induce reveromycin production in Streptomyces sp. SN-593". Scientific Reports 9 (1): 5802. April 2019. doi:10.1038/s41598-019-42268-w. PMID 30967594. Bibcode: 2019NatSR...9.5802P.
- ↑ "β-carboline chemical signals induce reveromycin production through a LuxR family regulator in Streptomyces sp. SN-593". Scientific Reports 10 (1): 10230. June 2020. doi:10.1038/s41598-020-66974-y. PMID 32576869. Bibcode: 2020NatSR..1010230P.
- ↑ "A small molecule produced by Lactobacillus species blocks Candida albicans filamentation by inhibiting a DYRK1-family kinase". Nature Communications 12 (1): 6151. October 2021. doi:10.1038/s41467-021-26390-w. PMID 34686660. Bibcode: 2021NatCo..12.6151M.
- ↑ 20.0 20.1 20.2 20.3 "Banisteriopsis caapi, a Forgotten Potential Therapy for Parkinson's Disease?". Movement Disorders Clinical Practice 3 (1): 19–26. 2016. doi:10.1002/mdc3.12242. PMID 30713897.
- ↑ "Beans, roots and leaves: a brief history of the pharmacological therapy of parkinsonism". Wurzburger Medizinhistorische Mitteilungen 22: 215–234. 2003. PMID 15641199.
- ↑ "Activities of extract and constituents of Banisteriopsis caapi relevant to parkinsonism". Pharmacology, Biochemistry, and Behavior 75 (3): 627–633. June 2003. doi:10.1016/s0091-3057(03)00129-1. PMID 12895680.
- ↑ "Harmine augments electrically evoked dopamine efflux in the nucleus accumbens shell". Journal of Psychopharmacology 27 (1): 98–108. January 2013. doi:10.1177/0269881112463125. PMID 23076833.
- ↑ "Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson's disease". Journal of Ethnopharmacology 127 (2): 357–367. February 2010. doi:10.1016/j.jep.2009.10.030. PMID 19879939.
- ↑ "DYRK1A promotes dopaminergic neuron survival in the developing brain and in a mouse model of Parkinson's disease". Cell Death & Disease 5 (6): e1289. June 2014. doi:10.1038/cddis.2014.253. PMID 24922073.
- ↑ "Harmaline competitively inhibits [3H]MK-801 binding to the NMDA receptor in rabbit brain". Brain Research 770 (1–2): 26–29. October 1997. doi:10.1016/s0006-8993(97)00606-9. PMID 9372198.
- ↑ The Encyclopedia of Psychoactive Plants: Ethnopharmacology and its Applications. Ratsch, Christian. Park Street Press c. 2005
- ↑ "Manganese dioxide mediated one-pot synthesis of methyl 9H-pyrido[3,4-bindole-1-carboxylate: Concise synthesis of alangiobussinine"]. Beilstein Journal of Organic Chemistry 7: 1407–1411. 2011. doi:10.3762/bjoc.7.164. PMID 22043251.
- ↑ "Partial least squares-based multivariate spectral calibration method for simultaneous determination of beta-carboline derivatives in Peganum harmala seed extracts". Analytica Chimica Acta 575 (2): 290–299. August 2006. doi:10.1016/j.aca.2006.05.093. PMID 17723604. Bibcode: 2006AcAC..575..290H.
- ↑ "beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO)". Food and Chemical Toxicology 48 (3): 839–845. March 2010. doi:10.1016/j.fct.2009.12.019. PMID 20036304.
- ↑ "Eudistomins from the New Zealand ascidian Ritterella sigillinoides". Aust. J. Chem. 42 (7): 1201–1206. 1989. doi:10.1071/CH9891201.
- ↑ "Eudistomin U and isoeudistomin U, new alkaloids from the Caribbean ascidian Lissoclinum fragile". Journal of Natural Products 57 (4): 528–533. April 1994. doi:10.1021/np50106a016. PMID 8021654.
- ↑ "Eudistomin V, a new beta-carboline from the Australian ascidian Pseudodistoma aureum". Journal of Natural Products 61 (7): 959–960. July 1998. doi:10.1021/np9800452. PMID 9677285.
- ↑ "Nostocarboline: isolation and synthesis of a new cholinesterase inhibitor from Nostoc 78-12A". Journal of Natural Products 68 (12): 1793–1795. December 2005. doi:10.1021/np050312l. PMID 16378379.
- ↑ Herraiz, Tomás (2011-11-10), "β-Carbolines as Neurotoxins", Isoquinolines And Beta-Carbolines As Neurotoxins And Neuroprotectants (Boston, MA: Springer US): pp. 77–103, doi:10.1007/978-1-4614-1542-8_5, ISBN 978-1-4614-1541-1, http://dx.doi.org/10.1007/978-1-4614-1542-8_5, retrieved 2021-11-16
- ↑ Herraiz, T.; González, D.; Ancín-Azpilicueta, C.; Arán, V.J.; Guillén, H. (March 2010). "β-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO)". Food and Chemical Toxicology 48 (3): 839–845. doi:10.1016/j.fct.2009.12.019. ISSN 0278-6915. PMID 20036304. http://dx.doi.org/10.1016/j.fct.2009.12.019.
- ↑ "The fluorescence of scorpions and cataractogenesis". Chemistry & Biology 6 (8): 531–539. August 1999. doi:10.1016/S1074-5521(99)80085-4. PMID 10421760.
- ↑ "Novel cytocidal compounds, oxopropalines from Streptomyces sp. G324 producing lavendamycin. I. Taxonomy of the producing organism, fermentation, isolation and biological activities". J. Antibiot. 46 (11): 1672–1677. 1993. doi:10.7164/antibiotics.46.1672. PMID 8270488.
External links
- Beta-Carbolines at the US National Library of Medicine Medical Subject Headings (MeSH)
- TiHKAL #44
- TiHKAL in general
- Beta-carbolines in coffee
- "Antidepressant-like effect of harmane and other beta-carbolines in the mouse forced swim test". European Neuropsychopharmacology 16 (5): 324–328. July 2006. doi:10.1016/j.euroneuro.2005.08.005. PMID 16183262.
{{Navbox | name = GABA receptor modulators | title = GABA receptor modulators | state = collapsed | bodyclass = hlist | groupstyle = text-align:center;
| group1 = Ionotropic | list1 = {{Navbox|subgroup | groupstyle = text-align:center | groupwidth = 5em
| group1 = GABAA | list1 =
- Agonists: (+)-Catechin
- Bamaluzole
- Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
- Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
- Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
- Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ | list2 =
- Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
- N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
- Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
- Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
- cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
- trans-3-ACPBPA
- ZAPA
- Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
- Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
}}
| group2 = Metabotropic
| list2 =
| below =
- See also
- Receptor/signaling modulators
- GABAA receptor positive modulators
- GABA metabolism/transport modulators
}}
 |