Chemistry:Naloxol
From HandWiki
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
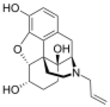
α-naloxol: (4R,4aS,7S,7aR,12bS)-3-allyl-2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-4a,7,9-triol
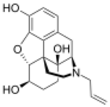
β-naloxol: (4R,4aS,7R,7aR,12bS)-3-allyl-2,3,4,4a,5,6,7,7a-octahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-4a,7,9-triol | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChemSpider | |||
PubChem CID
|
|||
| UNII |
| ||
| |||
| |||
| Properties | |||
| C19H23NO4 | |||
| Molar mass | 329.396 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Naloxol is an opioid antagonist closely related to naloxone. It exists in two isomeric forms, α-naloxol and β-naloxol.
α-naloxol is a human metabolite of naloxone.[1] Synthetically, α-naloxol can be prepared from naloxone by reduction of the ketone group, and β-naloxol can be prepared from α-naloxol by a Mitsunobu reaction.[2]
Naloxol can be said to be the oxymorphol analogue of naloxone.
See also
References
- ↑ Weinstein, S. H.; Pfeffer, M.; Schor, J. M.; Indindoli, L.; Mintz, M. (1971). "Metabolites of naloxone in human urine". Journal of Pharmaceutical Sciences 60 (10): 1567–1568. doi:10.1002/jps.2600601030. PMID 5129377.
- ↑ Simon, C (1994). "Stereoselective synthesis of β-naltrexol, β-naloxol β-naloxamine, β-naltrexamine and related compounds by the application of the mitsunobu reac". Tetrahedron 50 (32): 9757–9768. doi:10.1016/S0040-4020(01)85541-1.
 |