Chemistry:Dinalbuphine sebacate
 | |
| Clinical data | |
|---|---|
| Other names | DNS; Nalbuphine sebacate; Sebacoyldinalbuphine; SDN; Sebacoyl dinalbuphine ester; SDE; LT-1001 |
| Routes of administration | Intramuscular injection |
| Drug class | Opioid analgesic |
| Pharmacokinetic data | |
| Bioavailability | • IM: 85.4% (relative to nalbuphine)[1] |
| Metabolism | Hydrolysis[2] |
| Metabolites | Nalbuphine[1] |
| Elimination half-life | • DNS: 83.2 hours (mean absorption time: 145.2 hours)[1] • Nalbuphine: 4.0 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C52H68N2O10 |
| Molar mass | 881.120 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
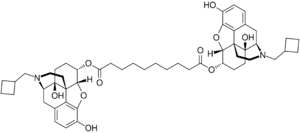
Dinalbuphine sebacate (DNS), also known as nalbuphine sebacate or as sebacoyl dinalbuphine ester (SDE) and sold under the brand name Naldebain, is a non-controlled opioid analgesic which is used as a 7-day long-acting injection in the treatment of moderate to severe postoperative pain.[3][4][5]
The compound is a diester of nalbuphine (Nubain) joined via a sebacic acid linker, and acts as a long-lasting prodrug of nalbuphine via slow hydrolysis.[5][2][6] It was developed to extend the duration of action of nalbuphine, which has a short duration and requires frequent injections.[7][5][2][1] Whereas nalbuphine must be injected every 4 to 6 hours, a single injection of DNS lasts for up to 7 to 10 days.[5]
It was invented by professor Oliver Yoa-Pu Hu (National Defense Medical Center) and codeveloped with Lumosa Therapeutics. Naldebain received market approvals from Taiwan FDA in March 2017, Health Sciences Authority of Singapore in December 2020, the Ministry of Public Health of Thailand in December 2021, the Drug Control Authority of Malaysia in 2022, State Service of Ukraine on Medicines and Drugs Control and Brunei Darussalam Medicines Control Authority (BDMCA) in 2023.[3][4] Development is ongoing in the United States , China, Korea, and the Philippines.[8]
Medical uses
Naldebain is indicated for the relief of moderate to severe acute postsurgical pain, administered intramuscularly. The product is available in single-use vials; 2 mL single use vial (75 mg/mL) for IM injection is packaged in a carton.[8]
Pharmacology
Pharmacodynamics
Nalbuphine, and hence DNS, acts as a mixed agonist/antagonist opioid modulator, or more specifically as a moderate-efficacy partial agonist or antagonist of the μ-opioid receptor and as a high-efficacy partial agonist of the κ-opioid receptor.[5][9][10][11][12]
Pharmacokinetics
The release mechanism of dinalbuphine sebacate (DNS) upon IM injection is as follows:[13]
- The injected Naldebain is first forming an oil depot in the muscle
- The oil depot gradually dispersed to small droplets in the surrounding tissues
- The prodrug DNS diffused out from the surface of oil droplets, and once released, a small portion of the prodrug get hydrolyzed by esterases to release the active ingredient, nalbuphine, in the surrounding tissue cells, while the majority of the prodrug would enter the blood stream through local tissue drainage (e.g., lymph flow) and get hydrolyzed to nalbuphine in the blood.
References
- ↑ 1.0 1.1 1.2 1.3 "Pharmacokinetics of dinalbuphine sebacate and nalbuphine in human after intramuscular injection of dinalbuphine sebacate in an extended-release formulation". Biopharmaceutics & Drug Disposition 38 (8): 494–497. November 2017. doi:10.1002/bdd.2088. PMID 28741675.
- ↑ 2.0 2.1 2.2 "In vitro and in vivo evaluation of the metabolism and pharmacokinetics of sebacoyl dinalbuphine". Drug Metabolism and Disposition 33 (3): 395–402. March 2005. doi:10.1124/dmd.104.002451. PMID 15608131.
- ↑ 3.0 3.1 "Dinalbuphine sebacate - Lumosa Therapeutics". AdisInsight. Springer Nature Switzerland AG. https://adisinsight.springer.com/drugs/800042506.
- ↑ 4.0 4.1 "Lumosa Therapeutics Partners with Camargo Pharmaceutical Services in the Development of Naldebain(R) in the US". www.prnewswire.com (Press release).
- ↑ 5.0 5.1 5.2 5.3 5.4 "Sebacoyl Dinalbuphine Ester Extended-release Injection for Long-acting Analgesia: A Multicenter, Randomized, Double-Blind, And Placebo-controlled Study in Hemorrhoidectomy Patients". The Clinical Journal of Pain 33 (5): 429–434. May 2017. doi:10.1097/AJP.0000000000000417. PMID 27518486.
- ↑ "High-performance liquid chromatographic method for the simultaneous determination of nalbuphine and its prodrug, sebacoyl dinalbuphine ester, in dog plasma and application to pharmacokinetic studies in dogs". Journal of Chromatography. B, Biomedical Sciences and Applications 746 (2): 241–247. September 2000. doi:10.1016/S0378-4347(00)00326-1. PMID 11076077.
- ↑ "Simultaneous determination of nalbuphine and its prodrug sebacoly dinalbuphine ester in human plasma by ultra-performance liquid chromatography-tandem mass spectrometry and its application to pharmacokinetic study in humans". Biomedical Chromatography 27 (7): 831–837. July 2013. doi:10.1002/bmc.2867. PMID 23460034.
- ↑ 8.0 8.1 "Lumosa Therapeutics" (in en). https://www.lumosa.com.tw/en/News/Pipeline/TIPO-Grant-LT1001-Formulation-Patent.
- ↑ "Nalbuphine, a non-controlled opioid analgesic, and its potential use in research mice". Lab Animal 44 (3): 106–110. March 2015. doi:10.1038/laban.701. PMID 25693108.
- ↑ "Nalbuphine". Drug and Alcohol Dependence 14 (3–4): 339–362. February 1985. doi:10.1016/0376-8716(85)90066-3. PMID 2986929.
- ↑ Clinical Anesthesia. Lippincott Williams & Wilkins. 2009. pp. 489–. ISBN 978-0-7817-8763-5. https://books.google.com/books?id=-YI9P2DLe9UC&pg=PA489.
- ↑ "Pharmacological properties of bivalent ligands containing butorphan linked to nalbuphine, naltrexone, and naloxone at mu, delta, and kappa opioid receptors". Journal of Medicinal Chemistry 50 (9): 2254–2258. May 2007. doi:10.1021/jm061327z. PMID 17407276.
- ↑ "BarashLumosa Therapeutics" (in en). https://www.lumosa.com.tw/en/Project/pipeline/2.
External links
 |


