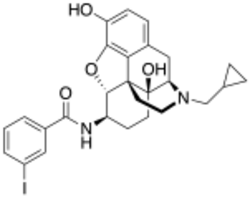
Chemistry:IBNtxA
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C27H29IN2O4 |
| Molar mass | 572.443 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
IBNtxA, or 3-iodobenzoyl naltrexamine, is an atypical opioid analgesic drug derived from naltrexone. In animal studies it produces potent analgesic effects that are blocked by levallorphan[1] and so appear to be μ-opioid mediated, but it fails to produce constipation or respiratory depression, and is neither rewarding or aversive[2] in conditioned place preference protocols.[3] These unusual properties are thought to result from agonist action at a splice variant or heterodimer of the μ-opioid receptor,[4] rather than at the classical full length form targeted by conventional opioid drugs.[5]
In the Radioligand binding assay it has shown to have affinities of 0.11nM at the MOR, 0.24nM at the DOR and 0.03nM at the KOR and in the Hot- Plate Assay it is shown to be around 20x more potent than morphine. Azido Aryl Analogues of IBNtxA retain significant activity at the MOR.[6]
References
- ↑ "Synthesis and evaluation of aryl-naloxamide opiate analgesics targeting truncated exon 11-associated μ opioid receptor (MOR-1) splice variants". J. Med. Chem. 55 (14): 6352–62. 2012. doi:10.1021/jm300305c. PMID 22734622.
- ↑ "Truncated G protein-coupled mu opioid receptor MOR-1 splice variants are targets for highly potent opioid analgesics lacking side effects". Proc. Natl. Acad. Sci. U.S.A. 108 (49): 19778–83. December 2011. doi:10.1073/pnas.1115231108. PMID 22106286. Bibcode: 2011PNAS..10819778M.
- ↑ "Pharmacologic characterization in the rat of a potent analgesic lacking respiratory depression, IBNtxA". J. Pharmacol. Exp. Ther. 350 (3): 710–8. 2014. doi:10.1124/jpet.114.213199. PMID 24970924.
- ↑ "Broad-spectrum analgesic efficacy of IBNtxA is mediated by exon 11-associated splice variants of the mu-opioid receptor gene". Pain 155 (10): 2063–70. 2014. doi:10.1016/j.pain.2014.07.014. PMID 25093831.
- ↑ Keck TM, Uddin MM, Babenko E, Wu C, Moura-Letts G. Abuse Liability and Anti-Addiction Potential of the Atypical Mu Opioid Receptor Agonist IBNtxA. The FASEB Journal 31 (1 Supplement), 985.4-985.4, 2017
- ↑ "Synthesis and Characterization of Azido Aryl Analogs of IBNtxA for Radio-Photoaffinity Labeling Opioid Receptors in Cell Lines and in Mouse Brain". Cell Mol Neurobiology 5 (41): 977–993. 2021. doi:10.1007/s10571-020-00867-6. PMID 32424771.
 |

