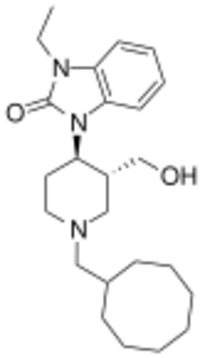
Chemistry:J-113,397
 | |
| Clinical data | |
|---|---|
| Other names | J-113,397 |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C24H37N3O2 |
| Molar mass | 399.579 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
J-113,397 is an opioid drug which was the first compound found to be a highly selective antagonist for the nociceptin receptor, also known as the ORL-1 receptor.[1][2] It is several hundred times selective for the ORL-1 receptor over other opioid receptors,[3][4] and its effects in animals include preventing the development of tolerance to morphine,[5] the prevention of hyperalgesia induced by intracerebroventricular administration of nociceptin (orphanin FQ),[6] as well as the stimulation of dopamine release in the striatum,[7] which increases the rewarding effects of cocaine,[8] but may have clinical application in the treatment of Parkinson's disease.[9][10][11]
Synthesis
Patents for treating arrhythmia:[12]

Condensation between 1-Benzyl-3-methoxycarbonyl-4-piperidone [57611-47-9] (1) and O-Phenylenediamine (2) gives CID:16726310 (3). Reaction with boc anhydride followed by treatment with trifluoroacetic acid gives CID:16726358 (4). Reaction with iodoethane in the presence of base alkylates the urea nitrogen giving CID:16726359 (5). Reduction of the enamine by treatment with magnesium metal in methanol solvent occurs to give predominantly the trans isomer, CID:16726360 (6). Catalytic removal of the benzyl group gives CID:16726362 (7). Reductive amination with Cyclooctanecarbaldehyde [6688-11-5] (7) gives CID:16726364 (9). Lastly, reduction of the ester with lithium aluminium hydride completed the synthesis of J-113397 (10).
See also
- JTC-801
- LY-2940094
- SB-612,111
- Trap-101 (unsaturated olefin not reduced).
References
- ↑ "Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3- hydroxymethyl-4-piperidyl]-3-ethyl-1, 3-dihydro-2H-benzimidazol-2-one (J-113397)". Journal of Medicinal Chemistry 42 (25): 5061–3. December 1999. doi:10.1021/jm990517p. PMID 10602690.
- ↑ "A new synthetic approach to 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-benzimidazol-2-one(J-113397), the first non-peptide ORL-1 receptor antagonist". Bioorganic & Medicinal Chemistry 9 (7): 1871–7. July 2001. doi:10.1016/s0968-0896(01)00085-2. PMID 11425589.
- ↑ "A potent and highly selective nonpeptidyl nociceptin/orphanin FQ receptor (ORL1) antagonist: J-113397". European Journal of Pharmacology 387 (3): R17-8. January 2000. doi:10.1016/s0014-2999(99)00822-5. PMID 10650183.
- ↑ "A new synthesis of the ORL-1 antagonist 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidinyl-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one (J-113397) and activity in a calcium mobilization assay"]. Bioorganic & Medicinal Chemistry 16 (2): 822–9. January 2008. doi:10.1016/j.bmc.2007.10.023. PMID 17976996.
- ↑ "Endogenous orphanin FQ/nociceptin is involved in the development of morphine tolerance". The Journal of Pharmacology and Experimental Therapeutics 318 (1): 262–7. July 2006. doi:10.1124/jpet.106.103960. PMID 16595734.
- ↑ "In vitro and in vivo pharmacological characterization of J-113397, a potent and selective non-peptidyl ORL1 receptor antagonist". European Journal of Pharmacology 402 (1–2): 45–53. August 2000. doi:10.1016/s0014-2999(00)00520-3. PMID 10940356.
- ↑ "Blockade of nociceptin/orphanin FQ receptor signaling in rat substantia nigra pars reticulata stimulates nigrostriatal dopaminergic transmission and motor behavior". The Journal of Neuroscience 24 (30): 6659–66. July 2004. doi:10.1523/JNEUROSCI.0987-04.2004. PMID 15282268.
- ↑ "The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine". Neuropharmacology 54 (3): 564–8. March 2008. doi:10.1016/j.neuropharm.2007.11.003. PMID 18082848.
- ↑ "The nociceptin/orphanin FQ receptor antagonist J-113397 and L-DOPA additively attenuate experimental parkinsonism through overinhibition of the nigrothalamic pathway". The Journal of Neuroscience 27 (6): 1297–307. February 2007. doi:10.1523/JNEUROSCI.4346-06.2007. PMID 17287504.
- ↑ "Nociceptin/orphanin FQ receptor blockade attenuates MPTP-induced parkinsonism". Neurobiology of Disease 30 (3): 430–8. June 2008. doi:10.1016/j.nbd.2008.02.011. PMID 18413287.
- ↑ "The nociceptin/orphanin FQ (NOP) receptor antagonist J-113397 enhances the effects of levodopa in the MPTP-lesioned nonhuman primate model of Parkinson's disease". Movement Disorders 23 (13): 1922–5. October 2008. doi:10.1002/mds.22086. PMID 18759357.
- ↑ Guo Zheng, et al. CN patent 111249279 & CN patent 111265663 (2020).
- ↑ "A New Approach to the Synthesis of the Nonpeptide NOP Receptor Antagonist J-113397". Synthesis 2007 (10): 1547–1553. 2 May 2007. doi:10.1055/s-2007-966037.
- ↑ Satoshi Ozaki, et al. WO patent 1998054168 (to MSD KK).
- ↑ Hiroshi Kawamoto, et al. WO patent 2000031061 (to MSD KK).
 |

