Chemistry:Pawhuskin A
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
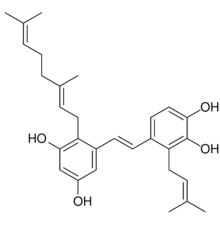
4-[(E)-2-{2-[(2E)-3,7-Dimethylocta-2,6-dien-1-yl]-3,5-dihydroxyphenyl}ethen-1-yl]-3-(3-methylbut-2-en-1-yl)benzene-1,2-diol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C29H36O4 | |
| Molar mass | 448.603 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pawhuskin A is a naturally occurring prenylated stilbene isolated from Dalea purpurea which acts as a competitive silent antagonist of the κ-, μ-, and δ-opioid receptors (Ke = 203 nM, 570 nM, and 2900 nM, respectively).[1][2][3] The compound was named after Pawhuska, Oklahoma, a place near where the samples of Dalea purpurea that led to its discovery were taken from.[3] Other isolates of the plant with affinity for opioid receptors include Pawhuskin B and Pawhuskin C, though these compounds produce comparatively weak opioid receptor displacement (4.2–11.4 μM) relative to Pawhuskin A.[1][2] Dalea purpurea was used in traditional Native American medicine to treat various ailments, and pawhuskin A and related isolates may be some of the constituents of the plant which underlay this use.[2]
See also
References
- ↑ 1.0 1.1 "New geranyl stilbenes from Dalea purpurea with in vitro opioid receptor affinity". J. Nat. Prod. 67 (1): 26–30. January 2004. doi:10.1021/np030258d. PMID 14738380.
- ↑ 2.0 2.1 2.2 "A concise synthesis of pawhuskin A". J. Nat. Prod. 71 (11): 1949–52. November 2008. doi:10.1021/np800351c. PMID 18922035.
- ↑ 3.0 3.1 "Stilbenes as κ-selective, non-nitrogenous opioid receptor antagonists". J. Nat. Prod. 77 (2): 311–9. February 2014. doi:10.1021/np4009046. PMID 24456556.
 |

