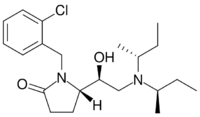
Chemistry:Viminol
 | |
| Clinical data | |
|---|---|
| Trade names | Dividol |
| Other names | Dividol, viminolo, diviminol |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H31ClN2O |
| Molar mass | 362.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
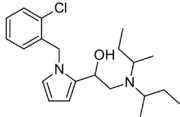
Viminol (marketed under the brandname Dividol) is an opioid analgesic developed by a team at the drug company Zambon in the 1960s.[1] Viminol is based on the α-pyrryl-2-aminoethanol structure, unlike any other class of opioids.[2][3]
Viminol has both antitussive (cough suppressing) and analgesic (pain reducing) effects. Viminol has additional effects similar to other opioids including sedation and euphoria.[citation needed] It has six different stereoisomers which have varying properties. Four are inactive, but the 1S-(R,R)-disecbutyl isomer is a μ-opioid full agonist around 5.5 times more potent than morphine and the 1S-(S,S)-disecbutyl isomer is an antagonist.[4][5] Since viminol is supplied as a racemic mixture of isomers, the overall effect is a mixed agonist–antagonist profile similar to that of opioids such as pentazocine, although with somewhat fewer side effects.[6]
Side effects
Side effects are similar to other opioids, and can include:
- Itching
- Nausea
- Sedation
- Respiratory depression - can be potentially life-threatening
However, since viminol is supplied as a racemic mixture of agonist and antagonist isomers, the abuse potential and respiratory depression tends to be less than that of μ-opioid full agonist drugs.
Drug dependence may occur.[7]
Related compounds
Later work showed that replacing the chlorine atom with an fluorine atom (2F-Viminol) or with a trifluoromethyl group produced a compound with twice the potency and half the acute toxicity.[8] A later team at Zambon found that one isomer of a pyrrolidone analog is 318 times as potent as morphine in its analgesic activity in animal studies.[9] A number of related compounds were also found to be active, allowing a QSAR model to be constructed.
References
- ↑ Teotino UM, Bella DD, "1-(α-Pyrryl)-2-amino Ethanols", US patent 3539589, issued 10 November 1970, assigned to Whitefin Holding SA
- ↑ "[Chromatographic separation of diastereoisomers of aminoalcohol salts and their densitometric determination]" (in Italian). Il Farmaco; Edizione Pratica 36 (4): 215–22. April 1981. PMID 6894429.
- ↑ "Psychopharmacological properties of the viminol-p-hydroxybenzoate". Revista Brasileira de Pesquisas Medicas e Biologicas 10 (6): 361–8. December 1977. PMID 609773.
- ↑ Della D, Bella CV, Monza DC, Tiotino UM, "Stereoisomers of 1(1'(-O-Chlorobenzyl)-2'-Pyrryl)-2-Disec.Butylamino-Ethanol", US patent 3857857, issued 31 December 1974, assigned to Whitefin Holding SA
- ↑ "The discriminative stimulus properties of the R2 isomer of viminol". Pharmacology, Biochemistry, and Behavior 20 (1): 59–62. January 1984. doi:10.1016/0091-3057(84)90101-1. PMID 6546450.
- ↑ "Viminol R2 analgesic activity in patients with postoperative pain: comparison with pentazocine". International Journal of Clinical Pharmacology, Therapy, and Toxicology 24 (5): 232–5. May 1986. PMID 3525423.
- ↑ "Dependence on Viminol". Journal of Substance Use 12 (4): 301–305. 2009. doi:10.1080/14659890701237124.
- ↑ Conti F, "Stereoisomers of 1-(1'benzyl-2'pyrryl)-2-di-sec.-butylaminoethanol and pharmaceutical compositions comprising same", US patent 4148907, issued 10 April 1979, assigned to Etablissement Viridis
- ↑ Carenzi A, Chiarino D, Bella DD, Grancini GC, Veneziani C, "Pyrrolidone-2 compounds and their use for central analgesic activity", US patent 4960788, issued 2 October 1990, assigned to Zambon Group S.P.A.
- ↑ "Stereoselective synthesis and evaluation of all stereoisomers of Z4349, a novel and selective μ-opioid analgesic.". Bioorganic & Medicinal Chemistry Letters 6 (5): 589–592. 1995. doi:10.1016/0960-894X(95)00077-7.
 |