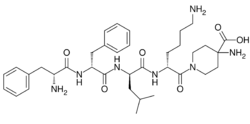
Chemistry:Difelikefalin
 | |
| Clinical data | |
|---|---|
| Trade names | Korsuva |
| Other names | CR845, FE-202845, D-Phe-D-Phe-D-Leu-D-Lys-[γ-(4-N-piperidinyl)amino carboxylic acid][1] |
| License data | |
| Pregnancy category | |
| Routes of administration | Intravenous |
| Drug class | Kappa opioid receptor agonist |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (IV)[8] |
| Metabolism | Not metabolized[8] |
| Elimination half-life | 2 hours[8] |
| Excretion | Excreted as unchanged drug via bile and urine[8] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C36H53N7O6 |
| Molar mass | 679.863 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
Difelikefalin, sold under the brand name Korsuva, is an opioid peptide used for the treatment of moderate to severe itch. It acts as a peripherally-restricted, highly selective agonist of the κ-opioid receptor (KOR).[8][9][10][11]
Difelikefalin acts as an analgesic by activating KORs on peripheral nerve terminals and KORs expressed by certain immune system cells.[8] Activation of KORs on peripheral nerve terminals results in the inhibition of ion channels responsible for afferent nerve activity, causing reduced transmission of pain signals, while activation of KORs expressed by immune system cells results in reduced release of proinflammatory, nerve-sensitizing mediators (e.g., prostaglandins).[8]
Difelikefalin was approved for medical use in the United States in August 2021.[6][12][13] The U.S. Food and Drug Administration considers it to be a first-in-class medication.[14]
Society and culture
Legal status
On 24 February 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Kapruvia, intended for treatment of moderate-to-severe pruritus associated with chronic kidney disease.[15] The applicant for this medicinal product is Vifor Fresenius Medical Care Renal Pharma France.[15] Difelikefalin was approved for medical use in the European Union in April 2022.[7][16]
Research
It is under development by Cara Therapeutics as an intravenous agent for the treatment of postoperative pain.[8][9][11] An oral formulation has also been developed.[11] Due to its peripheral selectivity, difelikefalin lacks the central side effects like sedation, dysphoria, and hallucinations of previous KOR-acting analgesics such as pentazocine and phenazocine.[8][9] In addition to use as an analgesic, difelikefalin is also being investigated for the treatment of pruritus (itching).[8][9][10] Difelikefalin has completed phase II clinical trials for postoperative pain and has demonstrated significant and "robust" clinical efficacy, along with being safe and well tolerated.[9][11] It has also completed a phase III clinical trial for uremic pruritus in hemodialysis patients.[17]
References
- ↑ "Development of opioid peptide analogs for pain relief". Current Pharmaceutical Design 16 (9): 1126–1135. 2010. doi:10.2174/138161210790963869. PMID 20030621.
- ↑ 2.0 2.1 "Korsuva". 25 November 2022. https://www.tga.gov.au/resources/auspmd/korsuva.
- ↑ "Updates to the Prescribing Medicines in Pregnancy database". 21 December 2022. https://www.tga.gov.au/resources/resource/guidance/updates-prescribing-medicines-pregnancy-database.
- ↑ "Product monograph". https://pdf.hres.ca/dpd_pm/00066996.PDF.
- ↑ "Summary Basis of Decision - Korsuva". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?lang=en&linkID=SBD00615&lang=en.
- ↑ 6.0 6.1 "Korsuva- difelikefalin injection, solution". https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0c7b81f2-0fd3-47cb-8b0f-185e07f19c87.
- ↑ 7.0 7.1 "Kapruvia EPAR". 22 February 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/kapruvia. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 "Peripheral kappa agonists". The Essence of Analgesia and Analgesics. Cambridge University Press. 14 October 2010. pp. 490–491. ISBN 978-1-139-49198-3. https://books.google.com/books?id=ZwPIjKg0XukC&pg=PA490.
- ↑ 9.0 9.1 9.2 9.3 9.4 Ambulatory Anesthesia, An Issue of Anesthesiology Clinics. Elsevier Health Sciences. 8 September 2014. pp. 190–. ISBN 978-0-323-29934-3. https://books.google.com/books?id=IlTOAwAAQBAJ&pg=PT190.
- ↑ 10.0 10.1 "Current Topical and Systemic Therapies for Itch". Pharmacology of Itch. Springer. 10 April 2015. pp. 307–. ISBN 978-3-662-44605-8. https://books.google.com/books?id=Jl0SCAAAQBAJ&pg=PA307.
- ↑ 11.0 11.1 11.2 11.3 "Oral Opioids". Pain Therapeutics: Current and Future Treatment Paradigms. Royal Society of Chemistry. 2013. pp. 56–. ISBN 978-1-84973-645-9. https://books.google.com/books?id=zUINAgAAQBAJ&pg=PA56.
- ↑ "Korsuva: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=214916.
- ↑ "Vifor Pharma and Cara Therapeutics announce U.S. FDA approval of Korsuva injection for the treatment of moderate-to-severe pruritus in hemodialysis patients" (Press release). Vifor Pharma. 24 August 2021. Retrieved 24 August 2021 – via Business Wire.
- ↑ (PDF) Advancing Health Through Innovation: New Drug Therapy Approvals 2021 (Report). 13 May 2022. https://www.fda.gov/media/155227/download. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 15.0 15.1 "Kapruvia: Pending EC decision". 24 February 2022. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/kapruvia. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Kapruvia Product information". https://ec.europa.eu/health/documents/community-register/html/h1643.htm.
- ↑ "A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus". The New England Journal of Medicine 382 (3): 222–232. January 2020. doi:10.1056/NEJMoa1912770. PMID 31702883.
External links
- "Difelikefalin". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/difelikefalin.
- Clinical trial number NCT03422653 for "A Study to Evaluate the Safety and Efficacy of CR845 in Hemodialysis Patients With Moderate-to-Severe Pruritus (KALM-1)" at ClinicalTrials.gov
- Clinical trial number NCT03636269 for "CR845-CLIN3103: A Global Study to Evaluate the Safety and Efficacy of CR845 in Hemodialysis Patients With Moderate-to-Severe Pruritus (KALM-2)" at ClinicalTrials.gov
 |
