Chemistry:Amastatin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| Chemical and physical data | |
| Formula | C21H38N4O8 |
| Molar mass | 474.555 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
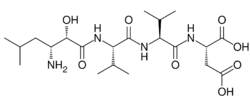
Amastatin, also known as 3-amino-2-hydroxy-5-methylhexanoyl-L-valyl-L-valyl-L-aspartic acid, is a naturally occurring, competitive and reversible aminopeptidase inhibitor that was isolated from Streptomyces sp. ME 98-M3.[1] It specifically inhibits leucyl aminopeptidase, alanyl aminopeptidase (aminopeptidase M/N), bacterial leucyl aminopeptidase (Aeromonas proteolytica aminopeptidase), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase),[2] and, to a lesser extent, glutamyl aminopeptidase (aminopeptidase A),[3] as well as other aminopeptidases.[4] It does not inhibit arginyl aminopeptidase (aminopeptidase B).[5][6] Amastatin has been found to potentiate the central nervous system effects of oxytocin and vasopressin in vivo.[7] It also inhibits the degradation of met-enkephalin, dynorphin A, and other endogenous peptides.[8]
See also
- Bestatin
- Pepstatin
References
- ↑ "Amastatins". Dictionary of Natural Products. CRC Press. 2 December 1993. pp. 197–. ISBN 978-0-412-46620-5. https://books.google.com/books?id=1W0NUD42fA4C&pg=PA197.
- ↑ "Immunoaffinity purification and characterization of native placental leucine aminopeptidase/oxytocinase from human placenta". Placenta 21 (7): 628–634. September 2000. doi:10.1053/plac.2000.0564. PMID 10985965.
- ↑ "Modern Aspects of Enzyme Inhibition with Particular Emphasis on Reaction-Intermediate Analogs and Other Potent, Reversible Inhibitors". Target Sites of Herbicide Action. CRC Press. 31 July 1989. pp. 203–. ISBN 978-0-8493-4985-0. https://books.google.com/books?id=eRGpF10Yj10C&pg=PA203.
- ↑ Concise Encyclopedia Biochemistry and Molecular Biology. Walter de Gruyter. 1997. pp. 35–. ISBN 978-3-11-014535-9. https://archive.org/details/conciseencyclope00scot_0.
- ↑ Small Molecular Immunomodifiers of Microbial Origin: Fundamental and Clinical Studies of Bestatin. Elsevier Science. 9 May 2014. pp. 10–. ISBN 978-1-4831-9033-4. https://books.google.com/books?id=p4XiBQAAQBAJ&pg=PA10.
- ↑ "Beta and Higher Homologous Amino Acids". Chemistry and Biochemistry of the Amino Acids. Springer Science & Business Media. 6 December 2012. pp. 28–. doi:10.1007/978-94-009-4832-7_3. ISBN 978-94-009-4832-7. https://books.google.com/books?id=gjLsCAAAQBAJ&pg=PA28.
- ↑ "Amastatin potentiates the behavioral effects of vasopressin and oxytocin in mice". Peptides 5 (3): 535–539. 1984. doi:10.1016/0196-9781(84)90083-4. PMID 6540873.
- ↑ "[Enkephalin-inactivating enzymes]" (in ja). Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica 101 (4): 197–207. April 1993. doi:10.1254/fpj.101.4_197. PMID 8390390.
 |
