Chemistry:Salicin

| |
| |
| Names | |
|---|---|
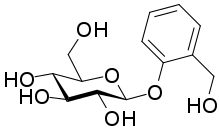
| IUPAC name
2-(Hydroxymethyl)phenyl β-D-glucopyranoside
| |
| Systematic IUPAC name
(2R,3S,4S,5R,6S)-2-(Hydroxymethyl)-6-[2-(hydroxymethyl)phenoxy]oxane-3,4,5-triol | |
| Other names
Salicin; D-(−)-Salicin; Salicoside
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | Glc(b)-O-Ph(2-CH2OH) |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C13H18O7 | |
| Molar mass | 286.280 g·mol−1 |
| Appearance | White crystals |
| Density | 1.434 g/cm3[2] |
| Melting point | 207 °C (405 °F; 480 K)[2] |
| Boiling point | 240 decomp.[2] |
| 43 g/L | |
| Solubility in Ethanol | 3 g/L |
| Solubility in DMSO | 20 g/L |
| Solubility in dimethyl formamide | 30 g/L |
| Hazards | |
| Main hazards | Skin sensitizer / Contact dermatitis[3] |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H317 | |
| P261, P272, P280, P302+352, P333+313, P362, P363, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Salicin is an alcoholic β-glucoside. Salicin is produced in (and named after) willow (Salix) bark. It is a biosynthetic precursor to salicylaldehyde.[4]
Medicinal aspects
Salicin is found in the bark of and leaves of willows, poplars and various other plants. Derivates are found in castoreum. Salicin from meadowsweet was used in the synthesis of aspirin (acetylsalicylic acid),[5] in 1899 by scientists at Bayer. Salicin tastes bitter like quinine.[6]
Salicin may cause an allergic skin reaction (skin sensitization; category 1).[3]
Mild side effects are standard, with rare occurrences of nausea, vomiting, rash, dizziness and breathing problems. Overdose from high quantities of salicin can be toxic, damaging kidneys, causing stomach ulcers, diarrhea, bleeding or digestive discomfort. Some people may be allergic or sensitive to salicylates and suffer reactions similar to those produced by aspirin. People should not take salicin if they have asthma, diabetes, gout, gastritis, hemophilia, stomach ulcers; also contraindicated are children under 16, and pregnant and breastfeeding women.[7]
References
- ↑ Merck Index, 11th Edition, 8293
- ↑ 2.0 2.1 2.2 Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 3.312. ISBN 9781498754293.
- ↑ 3.0 3.1 PubChem
- ↑ Pasteels, J. M.; Rowell-Rahier, M.; Braekman, J. C.; Dupont, A. (1983). "Salicin from host plant as precursor of salicylaldehyde in defensive secretion of Chrysomeline larvae". Physiological Entomology 8 (3): 307–314. doi:10.1111/j.1365-3032.1983.tb00362.x. https://dipot.ulb.ac.be/dspace/bitstream/2013/95098/4/ac2db4af-9a60-4c77-bdb4-6fa9e75d3003.txt.
- ↑ "History of Aspirin". http://inventors.about.com/library/inventors/blaspirin.htm.
- ↑ Daniells, S (2006-10-09). "Symrise explores cheaper alternatives in bitter-maskers". www.foodnavigator.com. http://www.foodnavigator.com/Science-Nutrition/Symrise-explores-cheaper-alternatives-in-bitter-maskers.
- ↑ "Willow bark | University of Maryland Medical Center". http://umm.edu/health/medical/altmed/herb/willow-bark.
 |


