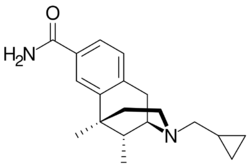
Chemistry:8-Carboxamidocyclazocine
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C19H26N2O |
| Molar mass | 298.430 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
8-Carboxamidocyclazocine (8-CAC) is an opioid analgesic drug related to cyclazocine, discovered by medicinal chemist Mark P. Wentland and co-workers in Cogswell Laboratory at Rensselaer Polytechnic Institute.[1] Similarly to cyclazocine, 8-CAC acts as an agonist at both the μ- and κ-opioid receptors, but has a much longer duration of action than cyclazocine, and does not have μ antagonist activity. Unexpectedly, it was discovered that the phenolic hydroxyl group of cyclazocine could be replaced by a carboxamido group with only slight loss of potency at opioid receptors, and this discovery has subsequently been used to develop many novel opioid derivatives where the phenolic hydroxy group has been replaced by either carboxamide or a variety of larger groups. Due to their strong κ-opioid agonist activity, these drugs are not suited for use as analgesics in humans, but have instead been researched as potential drugs for the treatment of cocaine addiction.[2][3][4][5][6][7][8][9][10]
See also
- Tianeptine – an atypical, selective μ-opioid receptor (MOR) full-agonist licensed for major depression since 1989.
- Samidorphan – an opioid preferring the MOR, which is under development for major depression.
References
- ↑ US Patent 6784187 8-carboxamido-2,6-methano-3-benzazocines
- ↑ "8-Carboxamidocyclazocine analogues: redefining the structure-activity relationships of 2,6-methano-3-benzazocines". Bioorganic & Medicinal Chemistry Letters 11 (5): 623–6. March 2001. doi:10.1016/S0960-894X(01)00014-2. PMID 11266156.
- ↑ "8-Carboxamidocyclazocine: a long-acting, novel benzomorphan". The Journal of Pharmacology and Experimental Therapeutics 302 (1): 374–80. July 2002. doi:10.1124/jpet.302.1.374. PMID 12065740.
- ↑ "Effects of the mixed-action kappa/mu opioid agonist 8-carboxamidocyclazocine on cocaine- and food-maintained responding in rhesus monkeys". European Journal of Pharmacology 506 (2): 133–41. December 2004. doi:10.1016/j.ejphar.2004.10.051. PMID 15588733.
- ↑ "Redefining the structure-activity relationships of 2,6-methano-3-benzazocines. 4. Opioid receptor binding properties of 8-[N-(4'-phenyl)-phenethyl)carboxamido] analogues of cyclazocine and ethylketocycalzocine". Journal of Medicinal Chemistry 49 (18): 5635–9. September 2006. doi:10.1021/jm060278n. PMID 16942039.
- ↑ "Redefining the structure-activity relationships of 2,6-methano-3-benzazocines. 5. Opioid receptor binding properties of N-((4'-phenyl)-phenethyl) analogues of 8-CAC". Bioorganic & Medicinal Chemistry Letters 17 (23): 6516–20. December 2007. doi:10.1016/j.bmcl.2007.09.082. PMID 17935988.
- ↑ "Redefining the structure-activity relationships of 2,6-methano-3-benzazocines. Part 6: Opioid receptor binding properties of cyclic variants of 8-carboxamidocyclazocine". Bioorganic & Medicinal Chemistry 16 (10): 5653–64. May 2008. doi:10.1016/j.bmc.2008.03.066. PMID 18417347.
- ↑ "Redefining the structure-activity relationships of 2,6-methano-3-benzazocines. Part 7: syntheses and opioid receptor properties of cyclic variants of cyclazocine". Bioorganic & Medicinal Chemistry Letters 19 (2): 365–8. January 2009. doi:10.1016/j.bmcl.2008.11.076. PMID 19091564.
- ↑ "Syntheses of novel high affinity ligands for opioid receptors". Bioorganic & Medicinal Chemistry Letters 19 (8): 2289–94. April 2009. doi:10.1016/j.bmcl.2009.02.078. PMID 19282177.
- ↑ "Kappa opioids as potential treatments for stimulant dependence". The AAPS Journal 7 (3): E592-9. October 2005. doi:10.1208/aapsj070361. PMID 16353938.
 |

