Chemistry:Proxorphan
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
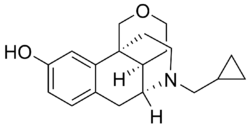
| Formula | C19H25NO2 |
| Molar mass | 299.414 g·mol−1 |
| 3D model (JSmol) | |
| |
Proxorphan (INN), also known as proxorphan tartate (USAN) (developmental code name BL-5572M), is an opioid analgesic and antitussive drug of the morphinan family that was never marketed.[1] It acts preferentially as a κ-opioid receptor partial agonist and to a lesser extent as a μ-opioid receptor partial agonist.[2][3][4][5][6]
Synthesis
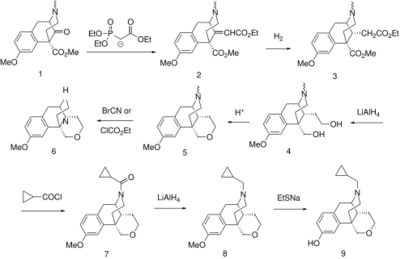
Starting material for this preparation is ketoester 1, available by one of the classical benzomorphan syntheses.[7] Condensation with the ylide from Triethyl phosphonoacetate (HWE reaction) affords diester 2. Catalytic hydrogenation proceeds from the less hindered face to afford the corresponding saturated diester (3). The esters are then reduced by means of LiAlH4 to give the glycol (4); this undergoes internal ether formation on treatment with acid to form the pyran ring of 5. Von Braun reaction with BrCN (or ethyl chloroformate) followed by saponification of the intermediate leads to the 2° amine (6). This is converted to the cyclopropylmethyl derivative 8 by acylation with cyclopropylcarbonyl chloride[8][9] followed by reduction of the thus formed amide (7) with LiAlH4. Cleaving off the O-methyl ether with sodium ethanethiol affords proxorphan (9).
See also
- Butorphanol
- Cyclorphan
- Ketorfanol
- Levallorphan
- Levomethorphan
- Levorphanol
- Moxazocine
- Nalbuphine
- Oxilorphan
- Xorphanol
References
- ↑ The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 1041–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA1041.
- ↑ "Further study of kappa opioids on increased urination". The Journal of Pharmacology and Experimental Therapeutics 227 (1): 35–41. October 1983. PMID 6137557. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=6137557.
- ↑ "Reversal by beta-funaltrexamine and 16-methyl cyprenorphine of the antinociceptive effects of opioid agonists in the mouse and guinea-pig". Neuropharmacology 27 (8): 813–816. August 1988. doi:10.1016/0028-3908(88)90096-2. PMID 3216959.
- ↑ "Discriminative stimulus effects of mu and kappa opioids in the pigeon: analysis of the effects of full and partial mu and kappa agonists". The Journal of Pharmacology and Experimental Therapeutics 249 (2): 557–566. May 1989. PMID 2566680. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=2566680.
- ↑ "Intermediate efficacy mu opioids: examination of their morphine-like stimulus effects and response rate-decreasing effects in morphine-tolerant rats". The Journal of Pharmacology and Experimental Therapeutics 263 (2): 668–681. November 1992. PMID 1331411. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=1331411.
- ↑ Advances in Drug Research. Elsevier. 22 October 2013. pp. 245–. ISBN 978-1-4832-8798-0. https://books.google.com/books?id=eiAlBQAAQBAJ&pg=PA245.
- ↑ "Syntheses in the morphine series; derivatives of bicyclo [3 : 3 : 1]-2-azanonane". Journal of the Chemical Society 169: 399–401. March 1947. doi:10.1039/JR9470000399. PMID 20240573.
- ↑ "Synthesis of cyclopropanecarbonyl chloride". Chemical Industry Times 17 (7): 36–38. 18 October 2018. http://caod.oriprobe.com/articles/6521356/Synthesis_of_Cyclopropanecarbonyl_chloride.htm.
- ↑ U.S. Patent 5,504,245
 |

