Chemistry:IC-26
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
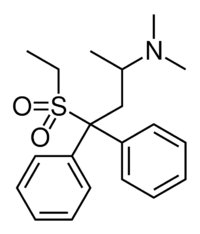
| Formula | C20H27NO2S |
| Molar mass | 345.50 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
IC-26[1] (WIN 1161-3, Methiodone)[2] is an analogue of the opioid analgesic methadone, where the carbonyl group has been replaced by the bioisosteric sulfone group.
Human and animal studies suggest that IC-26 is around the same potency as methadone,[3][4] although other studies have found its activity to be inconsistent between different patients, with consistent opioid activity only being seen at a dose several times that of methadone. IC-26 was assessed for its abuse potential, but despite being found to have similar potential to methadone for development of dependence[5] it was never placed under international control as an illegal drug.
See also
References
- ↑ "The Resolution of Ethyl 1,1-Diphenyl-3-dimethylaminobutyl Sulfone". Journal of the American Chemical Society 70 (11): 3959–3960. November 1948. doi:10.1021/ja01191a532. PMID 18207952.
- ↑ Archer S, Suter CM, Tullar BF, "Certain amino hydrocarbon sulfones and process of preparation", US patent 2618640, issued 1952-11-18, assigned to Sterling Drug
- ↑ Lednicer, D. (1982). Central Analgetics. Wiley. p. 194. ISBN 0-471-08314-3.
- ↑ "XVIII Sulphones". Diphenylpropylamines. Synthetic Analgesics. 1. Pergamon Press. 1960. pp. 160–163. https://www.scribd.com/doc/16407915/Synthetic-Analgesics-Vol-1-Diphenylpropylamines-Paul-Janssen-1960#page=166.
- ↑ "Addiction Liability of I-C-26". Bulletin on Narcotics (UNODC) 1963 (1): 25–28. 1963. https://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1963-01-01_1_page005.html.
 |

