Chemistry:Oxymorphazone
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Oxymorphone hydrazone |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
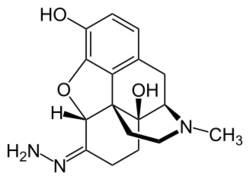
| Formula | C17H21N3O3 |
| Molar mass | 315.373 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Oxymorphazone is an opioid analgesic drug related to oxymorphone. Oxymorphazone is a potent and long acting μ-opioid agonist which binds irreversibly to the receptor, forming a covalent bond which prevents it from detaching once bound.[1][2] This gives it an unusual pharmacological profile, and while oxymorphazone is only around half the potency of oxymorphone, with higher doses the analgesic effect becomes extremely long lasting, with a duration of up to 48 hours.[3] However, tolerance to analgesia develops rapidly with repeated doses,[4][5][6] as chronically activated opioid receptors are rapidly internalised by β-arrestins, similar to the results of non-covalent binding by repeated doses of agonists with extremely high binding affinity such as lofentanil.[7][8]
See also
- Chlornaltrexamine, an irreversible mixed μ-opioid agonist-antagonist
- Chloroxymorphamine, another irreversible μ-opioid full agonist
- Naloxazone, an irreversible μ-opioid antagonist
References
- ↑ "Receptor binding and analgesic properties of oxymorphazone". Life Sciences 31 (12–13): 1389–92. 1982. doi:10.1016/0024-3205(82)90388-5. PMID 6183551.
- ↑ "Irreversible opiate agonists and antagonists: the 14-hydroxydihydromorphinone azines". The Journal of Neuroscience 2 (5): 572–6. May 1982. doi:10.1523/JNEUROSCI.02-05-00572.1982. PMID 6176696.
- ↑ "Discriminative stimulus effects of reversible and irreversible opiate agonists: morphine, oxymorphazone and buprenorphine". The Journal of Pharmacology and Experimental Therapeutics 230 (3): 652–7. September 1984. PMID 6206224.
- ↑ "Oxymorphazone: a long-acting opiate analgesic". Cellular and Molecular Neurobiology 4 (1): 1–13. March 1984. doi:10.1007/BF00710938. PMID 6204757.
- ↑ "Irreversible opiate agonists and antagonists. IV. Analgesic actions of 14-hydroxydihydromorphinone hydrazones". The Journal of Pharmacology and Experimental Therapeutics 245 (1): 8–12. April 1988. PMID 2452249.
- ↑ "Effects of oxymorphazone in frogs: long lasting antinociception in vivo, and apparently irreversible binding in vitro". Life Sciences 44 (24): 1847–57. 1989. doi:10.1016/0024-3205(89)90302-0. PMID 2472540.
- ↑ "Tracking the opioid receptors on the way of desensitization". Cellular Signalling 18 (11): 1815–33. November 2006. doi:10.1016/j.cellsig.2006.03.015. PMID 16750901.
- ↑ "Mechanisms of opioid-induced tolerance and hyperalgesia". Pain Management Nursing 8 (3): 113–21. September 2007. doi:10.1016/j.pmn.2007.02.004. PMID 17723928.
 |

