Chemistry:DAMGO
| |
| Names | |
|---|---|
| IUPAC name
(2S)-2-[[2-[[(2R)-2-[[(2S)-2-Amino-3-(4-hydroxyphenyl)propanoyl]amino]propanoyl]amino]acetyl]-methylamino]-N-(2-hydroxyethyl)-3-phenylpropanamide
| |
| Other names
Ala2-MePhe4-Glyol5-Enkephalin, DAGO, DAMGE
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C26H35N5O6 | |
| Molar mass | 513.595 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin) is a synthetic opioid peptide with high μ-opioid receptor specificity. It was synthesized as a biologically stable analog of δ-opioid receptor-preferring endogenous opioids, leu- and met-enkephalin.[1] Structures of DAMGO bound to the µ opioid receptor reveal a very similar binding pose to morphinans.[2][3]
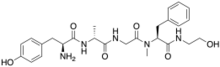
Its structure is H-Tyr-D-Ala-Gly-N-MePhe-Gly-ol.
DAMGO has been used in experimental settings for the possibility of alleviating or reducing opiate tolerance for patients under the treatment of an opioid. Such treatment on rats, adding DAMGO to morphine administration, showed that after seven days morphine had as much of an effect at the same dosage as the first day when administered together with DAMGO to the rats, whereas a separate control group of rats that were administered the same dosage of morphine over the course of the same week, but without DAMGO, displayed an increased tolerance and lessened analgesic efficacy toward the end of that week.[4][5][6]
See also
References
- ↑ "Analogues of β-LPH61–64 possessing selective agonist activity at μ-opiate receptors". European Journal of Pharmacology 70 (4): 531–40. April 1981. doi:10.1016/0014-2999(81)90364-2. PMID 6263640.
- ↑ "Structure of the µ-opioid receptor–Gi". Nature 558 (7711): 547–552. June 2018. doi:10.1038/s41586-018-0219-7. PMID 29899455.
- ↑ Zhuang, Youwen; Wang, Yue; He, Bingqing; He, Xinheng; Zhou, X. Edward; Guo, Shimeng; Rao, Qidi; Yang, Jiaqi et al. (2022-11-10). "Molecular recognition of morphine and fentanyl by the human μ-opioid receptor" (in en). Cell 185 (23): 4361–4375.e19. doi:10.1016/j.cell.2022.09.041. PMID 36368306.
- ↑ Radler, Don (25 January 2002). "Reducing Tolerance To Morphine Could Aid Pain Therapy". Daily University Science News (UniSci). UniScience News Net, Inc.. http://www.unisci.com/stories/20021/0125025.htm.
- ↑ "Endocytosis of the Mu Opioid Receptor Reduces Tolerance and a Cellular Hallmark of Opiate Withdrawal". Neuron 32 (5): 829–839. December 2001. doi:10.1016/S0896-6273(01)00517-7. PMID 11738029.
- ↑ "Regulation of Opioid Receptor Trafficking and Morphine Tolerance by Receptor Oligomerization". Cell 108 (2): 271–82. January 2002. doi:10.1016/S0092-8674(02)00613-X. PMID 11832216.
 |

