Chemistry:Norhydrocodone
From HandWiki
Short description: Chemical compound
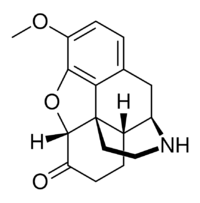 | |
| Clinical data | |
|---|---|
| Other names | (5α)-3-Methoxy-4,5-epoxymorphinan-6-one |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H19NO3 |
| Molar mass | 285.343 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Norhydrocodone is the major metabolite of the opioid analgesic hydrocodone.[1] It is formed from hydrocodone in the liver via N-demethylation predominantly by CYP3A4.[1] Unlike hydromorphone, a minor metabolite of hydrocodone, norhydrocodone is described as inactive.[2] However, norhydrocodone is actually an agonist of the μ-opioid receptor with similar potency to hydrocodone, but has been found to produce only minimal analgesia when administered peripherally to animals.[3] This is likely due to poor blood-brain-barrier and thus central nervous system penetration.[3]
See also
References
- ↑ 1.0 1.1 Cytochrome P450 2D6: Structure, Function, Regulation and Polymorphism. CRC Press. 6 April 2016. pp. 164–. ISBN 978-1-4665-9788-4. https://books.google.com/books?id=UJqmCwAAQBAJ&pg=PA164.
- ↑ Pharmacogenomics of Alcohol and Drugs of Abuse. CRC Press. 23 April 2012. pp. 175–. ISBN 978-1-4398-5611-6. https://books.google.com/books?id=AiHaRjs3grYC&pg=PA175.
 |

