Chemistry:Beta-Methylfentanyl
 | |
 | |
| Clinical data | |
|---|---|
| Other names | β-Methylfentanyl |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
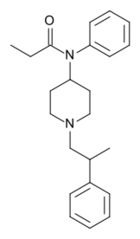
| Formula | C23H30N2O |
| Molar mass | 350.506 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
β-Methylfentanyl is an opioid analgesic that is an analogue of fentanyl.
β-Methylfentanyl was sold briefly on the black market in the early 1980s, before the introduction of the Federal Analog Act which, for the first time, attempted to control entire families of drugs based on their structural similarity rather than scheduling each drug individually as they appeared.[2]
β-Methylfentanyl has similar effects to fentanyl.[3] Side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[4]
References
- ↑ Drug Enforecement Administration, Department of Justice (February 2018). "Schedules of Controlled Substances:Temporary Placement of Fentanyl-Related Substances in Schedule I. Temporary amendment; temporary scheduling order". Federal Register 83 (25): 5188–92. PMID 29932611.
- ↑ "Designer drugs: past history and future prospects". Journal of Forensic Sciences 33 (2): 569–75. March 1988. doi:10.1520/JFS11976J. PMID 3286815.
- ↑ "3-Methylfentanyl". https://www.drugbank.ca/drugs/DB01571.
- ↑ "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". The International Journal on Drug Policy 26 (7): 626–31. July 2015. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511. http://www.ijdp.org/article/S0955-3959%2815%2900097-3/abstract.
