Chemistry:Clascoterone
 | |
| Clinical data | |
|---|---|
| Other names | CB-03-01; 11-Deoxycortisol 17α-propionate; 17α-(Propionyloxy)- deoxycorticosterone; 21-Hydroxy-3,20-dioxopregn-4-en-17-yl propionate |
| Routes of administration | Topical (cream) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
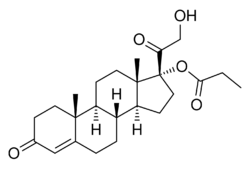
| Formula | C24H34O5 |
| Molar mass | 402.531 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Clascoterone, developmental code name CB-03-01 and tentative brand names Winlevi for acne and Breezula for hair loss, is an antiandrogen medication which is under development for the treatment of acne and scalp hair loss in both males and females.[1][2][3][4][5][6] It is given as a cream by application to the skin, for instance the face and scalp.[2]
Clascosterone is an antiandrogen, or antagonist of the androgen receptor (AR), the biological target of androgens such as testosterone and dihydrotestosterone.[7][8] It shows no systemic absorption when applied to skin.[2]
The medication is under development by Cassiopea and Intrepid Therapeutics.[1] As of 2019, it has completed phase III clinical trials for acne, with a New Drug Application expected for the first half of 2019, and is expected to start phase III clinical trials for androgen-dependent scalp hair loss.[1]
Medical uses
A pilot clinical trial in 2011 of men treated with topical clascoterone 1% cream for acne found that the medication significantly reduced symptoms of acne and was well tolerated.[4] Moreover, its effectiveness was significantly greater than that of the active comparator, tretinoin 0.05% cream.[4]
Pharmacology
Pharmacodynamics
Clascoterone is an steroidal antiandrogen, or antagonist of the androgen receptor (AR), the biological target of androgens such as testosterone and dihydrotestosterone (DHT).[7][8] In a bioassay, the topical potency of the medication was greater than that of progesterone, flutamide, and finasteride and was equivalent to that of cyproterone acetate.[3] Likewise, it is significantly more efficacious as an antiandrogen than other AR antagonists such as enzalutamide and spironolactone in scalp dermal papilla cells and sebocytes in vitro.[8]
Pharmacokinetics
In rodents, clascoterone has been found to possess strong local antiandrogenic activity, but negligible systemic antiandrogenic activity when administered via subcutaneous injection.[3] Along these lines, the medication is not progonadotropic in animals.[3]
Chemistry
Clascosterone, also known as cortexolone 17α-propionate or 11-deoxycortisol 17α-propionate, as well as 17α,21-dihydroxyprogesterone 17α-propionate or 17α,21-dihydroxypregn-4-en-3,20-dione 17α-propionate, is a synthetic pregnane steroid and a derivative of progesterone and 11-deoxycortisol (cortexolone).[9] It is specifically the C17α propionate ester of 11-deoxycortisol.[3]
An analogue of clascoterone is 9,11-dehydrocortexolone 17α-butyrate (CB-03-04).[10]
History
C17α esters of 11-deoxycortisol were unexpectedly found to possess antiandrogenic activity.[3] Clascoterone, also known as cortexolone 17α-propionate, was selected for development based on its optimal drug profile.[3]
Society and culture
Generic names
Clascoterone is the generic name of the drug and its INN and USAN.[9][11]
See also
- List of investigational hormonal agents § Androgenics
References
- ↑ 1.0 1.1 1.2 http://adisinsight.springer.com/drugs/800026561
- ↑ 2.0 2.1 2.2 "What's new in the management of acne vulgaris". Cutis 104 (1): 48–52. July 2019. PMID 31487336. https://www.mdedge.com/dermatology/article/204308/acne/whats-new-management-acne-vulgaris.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Biological profile of cortexolone 17alpha-propionate (CB-03-01), a new topical and peripherally selective androgen antagonist". Arzneimittelforschung 54 (12): 881–6. 2004. doi:10.1055/s-0031-1297043. PMID 15646372.
- ↑ 4.0 4.1 4.2 "Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. A pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream". Br. J. Dermatol. 165 (1): 177–83. 2011. doi:10.1111/j.1365-2133.2011.10332.x. PMID 21428978.
- ↑ "Cassiopea Announces Very Positive Top-Line Phase 3 Results for Winlevi® (Clascoterone) cream in Treating Acne" (in en). http://www.cassiopea.com/news-and-media/press-releases/yr-2018/180710.aspx.
- ↑ "Cassiopea Announces Very Positive Interim Analysis Phase 2 Results for Breezula® (Clascoterone) in Treating Androgenetic Alopecia" (in en). http://www.cassiopea.com/news-and-media/press-releases/yr-2018/180716.aspx.
- ↑ 7.0 7.1 "Cortexolone 17α-Propionate (Clascoterone) is an Androgen Receptor Antagonist in Dermal Papilla Cells In Vitro". J Drugs Dermatol 18 (2): 197–201. February 2019. PMID 30811143.
- ↑ 8.0 8.1 8.2 "Cortexolone 17α-propionate (Clascoterone) Is a Novel Androgen Receptor Antagonist that Inhibits Production of Lipids and Inflammatory Cytokines from Sebocytes In Vitro". J Drugs Dermatol 18 (5): 412–418. May 2019. PMID 31141847.
- ↑ 9.0 9.1 https://chem.nlm.nih.gov/chemidplus/rn/19608-29-8
- ↑ "Pharmacological profile of 9,11-dehydrocortexolone 17alpha-butyrate (CB-03-04), a new androgen antagonist with antigonadotropic activity". Arzneimittelforschung 55 (10): 581–7. 2005. doi:10.1055/s-0031-1296908. PMID 16294504.
- ↑ https://www.who.int/medicines/publications/druginformation/innlists/PL120.pdf
External links
 |

