Chemistry:Furethidine
From HandWiki
Short description: Chemical compound
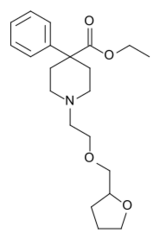 | |
| Clinical data | |
|---|---|
| Other names | ethyl 4-phenyl-1-(2-tetrahydrofurfuryloxyethyl)piperidine-4-carboxylate |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H31NO4 |
| Molar mass | 361.482 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Furethidine[1][2][3] is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine),[4] but with around 25x higher potency.[5] According to another source, Furethidine is 500/30 = 16.7 x the potency of pethidine (table VII).[6][7]
Furethidine is not currently used in medicine and is a Class A/Schedule I drug which is controlled under UN drug conventions. It has similar effects to other opioid derivatives, such as analgesia, sedation, nausea and respiratory depression.[8] In the United States it is a Schedule I Narcotic controlled substance with the ACSCN of 9626.[9]
References
- ↑ Frearson PM, Stern ES, "Novel piperidine compounds and their production", GB patent 797448, published 2 July 1958, assigned to J F Macfarlan & Co Ltd
- ↑ Frearson Peter Marshall; Stern Edward Severin, DE patent 1256219 (1967 to Glaxo Lab Ltd).
- ↑ "426. Some new analogues of pethidine. Part IV. Substituents at the 1-position incorporating cyclic ether groups". Journal of the Chemical Society (Resumed): 2103. 1960. doi:10.1039/jr9600002103. ISSN 0368-1769.
- ↑ "Opioids: 3.3 Synthetic Opioids.". Analgesics. Wiley-VCH. 2005. pp. 159–169. ISBN 978-3-527-30403-5.
- ↑ Casy AF, Parfitt RY. Opioid analgesics, chemistry and receptors. 1986, Plenum Press, New York. pp 234-235. ISBN 0-306-42130-5
- ↑ Blair, A. M. J. N.; Stephenson, R. P. (1960). "ANALGESIC ACTION OF ETHYL 4-PHENYLPIPERIDINE-4-CARBOXYLATES WITH OXYGENATED 1-SUBSTITUENTS". British Journal of Pharmacology and Chemotherapy. 15 (2): 247–253. doi:10.1111/j.1476-5381.1960.tb01239.x.
- ↑ Lister, R. E. (1960). "PHARMACOLOGICAL ACTIONS OF TWO NEW PETHIDINE ANALOGUES". British Journal of Pharmacology and Chemotherapy. 15 (2): 254–259. doi:10.1111/j.1476-5381.1960.tb01240.x.
- ↑ "A sequential trial of analgesics in labour". The Journal of Obstetrics and Gynaecology of the British Commonwealth 68: 88–93. February 1961. doi:10.1111/j.1471-0528.1961.tb02689.x. PMID 13689779.
- ↑ "Controlled Substances - Alphabetical Order". Diversion Control Division. Drug Enforcement Agency, U.S. Department of Justice. 2020. p. 9. https://www.deadiversion.usdoj.gov/schedules/orangebook/c_cs_alpha.pdf.
External links
 |
