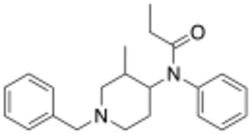
Chemistry:Isofentanyl
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C22H28N2O |
| Molar mass | 336.479 g·mol−1 |
| 3D model (JSmol) | |
| |
Isofentanyl (3-methyl-benzylfentanyl) is an opioid analgesic that is an analog (and structural isomer) of fentanyl first invented in 1973,[2] and which has been sold as a designer drug.[3]
Side effects
Side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[4] A new wave of fentanyl analogues and associated deaths began in around 2014 in the US, and have continued to grow in prevalence; especially since 2016 these drugs have been responsible for hundreds of overdose deaths every week.[5]
Legal status
As a structural isomer of fentanyl itself, isofentanyl is banned under drug analogue laws in many jurisdictions around the world. In the United States, fentanyl-related substances are Schedule I controlled substances.[1]
See also
- 3-Methylfentanyl
- Benzylfentanyl
- α-Methylacetylfentanyl
- 4-Methylphenethylacetylfentanyl
- Homofentanyl
- Secofentanyl
- List of fentanyl analogues
References
- ↑ 1.0 1.1 Drug Enforcement Administration, Department of Justice (2018). "Schedules of Controlled Substances:Temporary Placement of Fentanyl-Related Substances in Schedule I. Temporary amendment; temporary scheduling order". Federal Register 83 (25): 5188–92. PMID 29932611.
- ↑ "4-Anilidopiperidine analgesics. I. Synthesis and analgesic activity of certain ring-methylated 1-substituted 4-propananilidopiperidines". Journal of Pharmaceutical Sciences 62 (6): 983–6. June 1973. doi:10.1002/jps.2600620627. PMID 4712637.
- ↑ "Qualitative studies on the metabolism and the toxicological detection of the fentanyl-derived designer drugs 3-methylfentanyl and isofentanyl in rats using liquid chromatography-linear ion trap-mass spectrometry (LC-MS(n))". Analytical and Bioanalytical Chemistry 402 (3): 1249–55. January 2012. doi:10.1007/s00216-011-5528-8. PMID 22065349.
- ↑ "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". The International Journal on Drug Policy 26 (7): 626–31. July 2015. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511. http://www.ijdp.org/article/S0955-3959%2815%2900097-3/abstract.
- ↑ "Fentanyl, fentanyl analogs and novel synthetic opioids: A comprehensive review". Neuropharmacology 134 (Pt A): 121–132. October 2017. doi:10.1016/j.neuropharm.2017.10.016. PMID 29042317. https://cloudfront.escholarship.org/dist/prd/content/qt8xh0s7nf/qt8xh0s7nf.pdf.
 |
