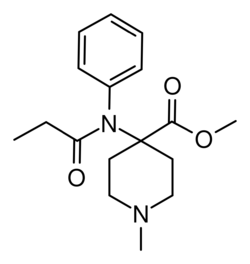
Chemistry:N-Methylcarfentanil
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H24N2O3 |
| Molar mass | 304.384 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
N-Methylcarfentanil (R-32395) is an opioid analgesic drug related to the highly potent animal tranquilizer carfentanil, but several thousand times weaker, being only slightly stronger than morphine. It was first synthesised by a team of chemists at Janssen Pharmaceutica led by Paul Janssen, who were investigating the structure-activity relationships of the fentanyl family of drugs. They found that replacing the phenethyl group attached to the piperidine nitrogen of fentanyl with a smaller methyl group, made it so much weaker that it was inactive as an analgesic in animals. However the same change made to the more potent analogue carfentanil retained reasonable opioid receptor activity, reflecting the higher binding affinity produced by the 4-carbomethoxy group.[1][2][3]
Side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[4]
References
- ↑ "Molecular determinants of mu receptor recognition for the fentanyl class of compounds.". Molecular Pharmacology 41 (1): 185–196. January 1992. PMID 1310142. http://molpharm.aspetjournals.org/content/41/1/185.abstract.
- ↑ Maguire, P.; Tsai, N.; Kamal, J.; Cometta-Morini, C.; Upton, C.; Loew, G. (1992). "Pharmacological profiles of fentanyl analogs at μ, δ and κ opiate receptors". European Journal of Pharmacology 213 (2): 219–225. doi:10.1016/0014-2999(92)90685-W. PMID 1355735. http://www.sciencedirect.com/science/article/pii/001429999290685W.
- ↑ Dukhovich FS, Darkhovskii MB, Gorbatova EN, Kurochkin VK. Molecular recognition: pharmacological aspects. 2004, Nova Science Publishers, New York. p 81. ISBN:1-59033-887-1
- ↑ Jane Mounteney; Isabelle Giraudon; Gleb Denissov; Paul Griffiths (July 2015). "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". International Journal of Drug Policy 26 (7): 626–631. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511. http://www.sciencedirect.com/science/article/pii/S0955395915000973.

