Chemistry:Pericine
From HandWiki
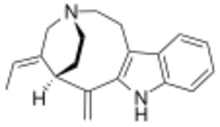
| |
| Names | |
|---|---|
| IUPAC name
(1R,16E)-16-Ethylidene-2-methylene-4,14-diazatetracyclo[12.2.2.03,11.05,10]octadeca-3(11),5,7,9-tetraene
| |
| Other names
Subincanadine E
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H22N2 | |
| Molar mass | 278.399 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pericine is one of a number of indole alkaloids found in the tree Picralima nitida, commonly known as akuamma. As with some other alkaloids from this plant such as akuammine, pericine has been shown to bind to mu opioid receptors in vitro, and has an IC50 of 0.6 μmol, within the range of a weak analgesic.[1] It may also have convulsant effects.[2]
Pericine has been prepared in the laboratory by total synthesis.[3][4]
See also
References
- ↑ "Detection of pericine, a new CNS-active indole alkaloid from Picralima nitida cell suspension culture by opiate receptor binding studies". Planta Medica 46 (4): 210–4. December 1982. doi:10.1055/s-2007-971216. PMID 6298847.
- ↑ Alkaloids: Biochemistry, Ecology, and Medicinal Applications. Springer. 30 June 1998. pp. 68–69. ISBN 978-0-306-45465-3.
- ↑ "Total synthesis of indole alkaloid (±)-subincanadine E". Organic Letters 16 (12): 3173–5. June 2014. doi:10.1021/ol501308p. PMID 24869784.
- ↑ "Total Synthesis of (±)/(+)-Subincanadine E and Determination of Absolute Configuration". The Journal of Organic Chemistry 82 (20): 11126–11133. October 2017. doi:10.1021/acs.joc.7b02122. PMID 28952728.
 |

