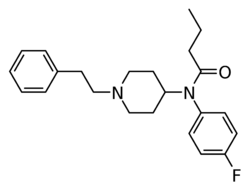
Chemistry:4-Fluorobutyrfentanyl
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C23H29FN2O |
| Molar mass | 368.496 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
4-Fluorobutyrylfentanyl (also known as 4-FBF and p-FBF or para-fluorobutyrylfentanyl) is an opioid analgesic that is an analog of butyrfentanyl and has been sold online as a designer drug.[1][2] It is closely related to 4-fluorofentanyl, which has an EC50 value of 4.2 nM for the human μ-opioid receptor.[3]
Side effects
Side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[4]
Legal status
Sweden's public health agency suggested classifying 4-fluorobutyrylfentanyl as hazardous substance on August 18, 2014.[5]
In October 2015, 4-fluorobutyrylfentanyl became a controlled substance in China.[6]
4-Fluorobutyrfentanyl is a Schedule I controlled drug in the USA since 1. February 2018.[7]
See also
- 3-Methylbutyrfentanyl
- 3-Methylfentanyl
- 4-Fluorofentanyl
- 4-Fluoroisobutyrfentanyl
- α-Methylfentanyl
- Acetylfentanyl
- Acrylfentanyl
- Furanylfentanyl
- List of fentanyl analogues
References
- ↑ "para-Fluorobutyrylfentanyl". Cayman Chemical. https://www.caymanchem.com/Product.vm/catalog/17049.
- ↑ "Opioid intoxications involving butyrfentanyl, 4-fluorobutyrfentanyl, and fentanyl from the Swedish STRIDA project". Clinical Toxicology 53 (7): 609–17. 9 August 2015. doi:10.3109/15563650.2015.1054505. PMID 26083809.
- ↑ "Interaction of p-fluorofentanyl on cloned human opioid receptors and exploration of the role of Trp-318 and His-319 in mu-opioid receptor selectivity". The Journal of Pharmacology and Experimental Therapeutics 294 (3): 1024–33. September 2000. PMID 10945855. http://jpet.aspetjournals.org/content/294/3/1024.full.
- ↑ "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". The International Journal on Drug Policy 26 (7): 626–31. July 2015. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.
- ↑ "Nya substanser klassas som narkotika eller hälsofarlig vara" (in sv). Folkhälsomyndigheten. 18 August 2015. https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2015/augusti/nya-substanser-klassas-som-narkotika-eller-halsofarlig-vara/.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "Schedules of Controlled Substances: Temporary Placement of Seven Fentanyl-Related Substances in Schedule I". Federal Register. 1 February 2018. https://www.federalregister.gov/documents/2018/02/01/2018-02008/schedules-of-controlled-substances-temporary-placement-of-seven-fentanyl-related-substances-in.
 |

