Chemistry:EPI-001
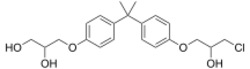 | |
| Clinical data | |
|---|---|
| Drug class | Nonsteroidal antiandrogen |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H27ClO5 |
| Molar mass | 394.89 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
EPI-001 is the first inhibitor of the androgen receptor amino-terminal domain. The single stereoisomer of EPI-001, EPI-002, is a first-in-class drug that the USAN council assigned a new stem class "-aniten" and the generic name "ralaniten". This distinguishes the anitens novel molecular mechanism from anti androgens that bind the C-terminus ligand-binding domain and have the stem class "lutamide" (such as flutamide, nilutamide, bicalutamide, enzalutamide, etc.). EPI-001 and its stereoisomers and analogues were discovered by Marianne Sadar and Raymond Andersen, who co-founded the pharmaceutical company ESSA Pharma Inc (Vancouver, Canada) for the clinical development of anitens for the treatment of castration-resistant prostate cancer (CRPC).
EPI-001 is an antagonist of the androgen receptor (AR) that acts by binding covalently to the N-terminal domain (NTD) of the AR and blocking protein-protein interactions required for transcriptional activity of the AR and its splice variants (IC50 for inhibition of AR NTD transactivation ≈ 6 μM).[1][2] This is different from all currently-used antiandrogens, which, conversely, bind to the C-terminal ligand-binding domain (LBD) of the AR and competitively block binding and activation of the receptor by androgens.[1] Due to its unique mechanism of action, EPI-001 type compounds may prove to be effective in the treatment of advanced prostate cancer resistant to conventional antiandrogens such as enzalutamide.[1]
EPI-001's successor, ralaniten acetate (EPI-506), a prodrug of ralaniten (EPI-002), one of the four stereoisomers of EPI-001, was under clinical investigation in a phase I study.[3] EPI-506 was the first drug that directly binds to an intrinsically disordered region to be tested in humans and marks a leap in drug development from folded drug targets.
Pharmacology
Pharmacodynamics
EPI-001 is a mixture of four stereoisomers. EPI-001 binds to the activation function-1 (AF-1) region in the NTD of the AR, as opposed to other AR antagonists, which bind to the C-terminal LBD.[4] A functional AF-1 is essential for the AR to have transcriptional activity. If AF-1 is deleted or mutated, the AR will still bind androgens, but will have no transcriptional activity.[5] Importantly, if the AR lacks an LBD, the receptor will be nuclear and constitutively-active.[5] Constitutively active splice variants of the AR that lack the C-terminal LBD are correlated to CRPC and poor survival.[6][7][8][9][10][11] EPI-001 is an inhibitor of constitutively active splice variant of ARs that lack the C-terminal LBD.[2] Conventional antiandrogens do not inhibit constitutively-active variants of AR that have a truncated or deleted C-terminal LBD.
In the absence of androgen, all known antiandrogens cause translocation of AR from the cytoplasm to the nucleus,[4][12][13] whereas EPI-001 does not cause the AR to become nuclear.[2] Binding of EPI-001 to the NTD of the AR blocks protein-protein interactions that are essential for its transcriptional activity. Specifically, EPI-001 blocks AR interactions with CREB-binding protein, RAP74, and between the NTD and C-terminal domain (termed N/C interaction) required for antiparallel dimer formation of AR.[2] Unlike antiandrogens such as bicalutamide,[12][14] EPI-001 does not cause the AR to bind to androgen response elements on the DNA of target genes.[2]
EPI-001 at extremely high concentrations of 50 to 200 uM has also been found to act as a selective PPARγ modulator (SPPARM), with both agonistic and antagonistic actions on the PPARγ.[15] Via PPARγ activation, EPI-001 has been found to inhibit AR expression and activity in prostate cancer cells, indicating at least one AR-independent action by which EPI-001 exhibits antiandrogen properties in the prostate.[15]
EPI-001 inhibits AR-dependent proliferation of human prostate cancer cells while having no significant effects on cells that do not require the AR for growth and survival.[2] EPI-001 has specificity to the AR (aside from the PPARγ) and has excellent anti-tumor activity in vivo with xenografts of CRPC.[2]
See also
- EPI-002
- EPI-7386
References
- ↑ 1.0 1.1 1.2 "New agents for prostate cancer". Annals of Oncology 25 (9): 1700–1709. September 2014. doi:10.1093/annonc/mdu038. PMID 24658665.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor". Cancer Cell 17 (6): 535–546. June 2010. doi:10.1016/j.ccr.2010.04.027. PMID 20541699.
- ↑ "Safety and Anti-Tumor Study of Oral EPI-506 for Patients With Metastatic Castration-Resistant Prostate Cancer - Full Text View - ClinicalTrials.gov". https://clinicaltrials.gov/ct2/show/NCT02606123.
- ↑ 4.0 4.1 "Small molecule inhibitors targeting the "achilles' heel" of androgen receptor activity". Cancer Research 71 (4): 1208–1213. February 2011. doi:10.1158/0008-5472.CAN_10-3398. PMID 21285252.
- ↑ 5.0 5.1 "Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization". Molecular Endocrinology 5 (10): 1396–1404. October 1991. doi:10.1210/mend-5-10-1396. PMID 1775129.
- ↑ "A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth". Cancer Research 69 (6): 2305–2313. March 2009. doi:10.1158/0008-5472.can-08-3795. PMID 19244107.
- ↑ "Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer". Cancer Research 69 (1): 16–22. January 2009. doi:10.1158/0008-5472.can-08-2764. PMID 19117982.
- ↑ "Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant". The Journal of Clinical Investigation 120 (8): 2715–2730. August 2010. doi:10.1172/jci41824. PMID 20644256.
- ↑ "Androgen receptor and its splice variants in prostate cancer". Cellular and Molecular Life Sciences 68 (24): 3971–3981. December 2011. doi:10.1007/s00018-011-0766-7. PMID 21748469.
- ↑ "Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival". PLOS ONE 6 (4): e19059. April 2011. doi:10.1371/journal.pone.0019059. PMID 21552559. Bibcode: 2011PLoSO...619059H.
- ↑ "Androgen receptor variants occur frequently in castration resistant prostate cancer metastases". PLOS ONE 6 (11): e27970. 2011. doi:10.1371/journal.pone.0027970. PMID 22114732. Bibcode: 2011PLoSO...627970Z.
- ↑ 12.0 12.1 "ARN-509: a novel antiandrogen for prostate cancer treatment". Cancer Research 72 (6): 1494–1503. March 2012. doi:10.1158/0008-5472.CAN-11-3948. PMID 22266222.
- ↑ "Advances in small molecule inhibitors of androgen receptor for the treatment of advanced prostate cancer". World Journal of Urology 30 (3): 311–318. June 2012. doi:10.1007/s00345-011-0745-5. PMID 21833557.
- ↑ "Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor". The Journal of Biological Chemistry 277 (29): 26321–26326. July 2002. doi:10.1074/jbc.M203310200. PMID 12015321.
- ↑ 15.0 15.1 "EPI-001 is a selective peroxisome proliferator-activated receptor-gamma modulator with inhibitory effects on androgen receptor expression and activity in prostate cancer". Oncotarget 6 (6): 3811–3824. February 2015. doi:10.18632/oncotarget.2924. PMID 25669987.
{{Navbox
| name = Androgens and antiandrogens | title = Androgens and antiandrogens | state = collapsed | listclass = hlist | groupstyle = text-align:center;
| group1 = Androgens
(incl. AAS)
| list1 =
| group2 = Antiandrogens | list2 = {{Navbox|child | groupstyle = text-align:center; | groupwidth = 9em;
| group1 = AR antagonists | list1 =
- Steroidal: Abiraterone acetate
- Canrenone
- Chlormadinone acetate
- Cyproterone acetate
- Delmadinone acetate
- Dienogest
- Drospirenone
- Medrogestone
- Megestrol acetate
- Nomegestrol acetate
- Osaterone acetate
- Oxendolone
- Potassium canrenoate
- Spironolactone
- Nonsteroidal: Apalutamide
- Bicalutamide
- Cimetidine
- Darolutamide
- Enzalutamide
- Flutamide
- Ketoconazole
- Nilutamide
- Seviteronel†
- Topilutamide (fluridil)
| group2 = Steroidogenesis| list2 =
inhibitors
| 5α-Reductase | |
|---|---|
| Others |
| group3 = Antigonadotropins | list3 =
- D2 receptor antagonists (prolactin releasers) (e.g., domperidone, metoclopramide, risperidone, haloperidol, chlorpromazine, sulpiride)
- Estrogens (e.g., bifluranol, [[diethylstilbestrol, estradiol, estradiol esters, ethinylestradiol, ethinylestradiol sulfonate, paroxypropione)
- GnRH agonists (e.g., leuprorelin)
- GnRH antagonists (e.g., cetrorelix)
- Progestogens (incl., chlormadinone acetate, [[cyproterone acetate, hydroxyprogesterone caproate, gestonorone caproate, [[Chemistry:Medroxyprogesterone medroxyprogesterone acetate, Chemistry:Megestrol acetate|megestrol acetate]])
| group4 = Others | list4 =
- Androstenedione immunogens: Androvax (androstenedione albumin)
- Ovandrotone albumin (Fecundin)
}}
| liststyle = background:#DDDDFF;| list3 =
- #WHO-EM
- ‡Withdrawn from market
- Clinical trials:
- †Phase III
- §Never to phase III
- See also
- Androgen receptor modulators
- Estrogens and antiestrogens
- Progestogens and antiprogestogens
- List of androgens/anabolic steroids
}}
 |

