Chemistry:SC-17599
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
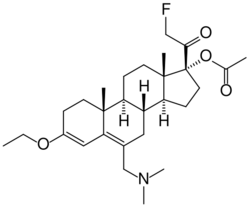
| Other names | SC-17599; 17α-Acetoxy-6-dimethylaminomethyl-21-fluoro-3-ethoxypregna-3,5-dien-20-one; [(8R,9S,10R,13S,14S,17R)-6-(dimethylaminomethyl)-3-ethoxy-17-(2-fluoroacetyl)-10,13-dimethyl-1,2,7,8,9,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl] acetate |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C28H42FNO4 |
| Molar mass | 475.645 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
SC-17599 is a steroid derivative drug discovered in 1968 which acts as a selective μ-opioid receptor agonist, with little or no affinity for the δ-opioid or κ-opioid receptors. It is an active analgesic in vivo, more potent than codeine or pethidine but slightly less potent than morphine, [1] and produces similar effects to morphine in animals but with less sedation[2][3]
See also
References
- ↑ "Analgetic Activity and Other Pharmacological Properties of a Steroid; 17α-acetoxy-6-dimethylaminomethyl-21-fluoro-3-ethoxypregna-3,5-dien-20-one Hydrochloride (SC17599)". Journal of Pharmacology and Experimental Therapeutics 164 (2): 371–379. 1968. PMID 5699098.
- ↑ "The steroid 17alpha-acetoxy-6-dimethylaminomethyl-21-fluoro-3-ethoxy-pregna-3, 5-dien-20-one (SC17599) is a selective mu-opioid agonist: implications for the mu-opioid pharmacophore". Molecular Pharmacology 58 (4): 669–76. October 2000. doi:10.1124/mol.58.4.669. PMID 10999935.
- ↑ "Antinociceptive and other behavioral effects of the steroid SC17599 are mediated by the mu-opioid receptor". European Journal of Pharmacology 395 (2): 121–8. April 2000. doi:10.1016/s0014-2999(00)00176-x. PMID 10794817.
 |

