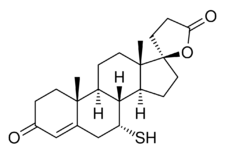
Chemistry:7α-Thiospironolactone
 | |
| Clinical data | |
|---|---|
| Other names | 7α-TS; SC-24813; Deacetylspironolactone; Mercaptospironolactone; 17α-Hydroxy-7α-mercapto-3-oxopregn-4-ene-21-carboxylic acid γ-lactone |
| Drug class | Antimineralocorticoid; Antiandrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H30O3S |
| Molar mass | 374.54 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
7α-Thiospironolactone (7α-TS; developmental code name SC-24813; also known as deacetylspironolactone) is a steroidal antimineralocorticoid and antiandrogen of the spirolactone group and a minor active metabolite of spironolactone.[1][2] Other important metabolites of spironolactone include 7α-thiomethylspironolactone (7α-TMS; SC-26519), 6β-hydroxy-7α-thiomethylspironolactone (6β-OH-7α-TMS), and canrenone (SC-9376).[1][2][3][4]
Spironolactone is a prodrug with a short terminal half-life of 1.4 hours.[5][6][7] The active metabolites of spironolactone have extended terminal half-lives of 13.8 hours for 7α-TMS, 15.0 hours for 6β-OH-7α-TMS, and 16.5 hours for canrenone, and accordingly, these metabolites are responsible for the therapeutic effects of the drug.[5][6]
7α-TS and 7α-TMS have been found to possess approximately equivalent affinity for the rat ventral prostate androgen receptor (AR) relative to that of spironolactone.[8] The affinity of 7α-TS, 7α-TMS, and spironolactone for the rat prostate AR is about 3.0 to 8.5% of that of dihydrotestosterone (DHT).[8]
7α-TS, via a reactive metabolite formed by 17α-hydroxylase, is a suicide inhibitor of 17α-hydroxylase, and is thought to be involved in the inhibition of 17α-hydroxylase by spironolactone.[9][10][11]
| Compound | Cmax (ng/mL) (day 1) |
Cmax (ng/mL) (day 15) |
AUC (ng•hr/ml) (day 15) |
t1/2 (hr) |
|---|---|---|---|---|
| Spironolactone | 72 | 80 | 231 | 1.4 |
| Canrenone | 155 | 181 | 2173 | 16.5 |
| 7α-TMS | 359 | 391 | 2804 | 13.8 |
| 6β-OH-7α-TMS | 101 | 125 | 1727 | 15.0 |
A study assessed the interaction of spironolactone and 7α-TS with sex hormone-binding globulin and found that they had very low affinity for this carrier protein.[13]
See also
References
- ↑ 1.0 1.1 "Mineralocorticoid receptor antagonists". Curr. Hypertens. Rep. 9 (1): 45–52. 2007. doi:10.1007/s11906-007-0009-3. PMID 17362671.
- ↑ 2.0 2.1 "30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development". J. Endocrinol. 234 (1): T125–T140. 2017. doi:10.1530/JOE-16-0600. PMID 28634268.
- ↑ "Mineralocorticoid receptor antagonists-pharmacodynamics and pharmacokinetic differences". Curr Opin Pharmacol 27: 78–85. 2016. doi:10.1016/j.coph.2016.02.005. PMID 26939027.
- ↑ "The spironolactone renaissance". Expert Opin Investig Drugs 10 (5): 943–54. 2001. doi:10.1517/13543784.10.5.943. PMID 11322868.
- ↑ 5.0 5.1 "Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis". Heart Fail Rev 10 (1): 23–9. 2005. doi:10.1007/s10741-005-2345-1. PMID 15947888.
- ↑ 6.0 6.1 "Mineralocorticoid receptor antagonists and endothelial function". Curr Opin Investig Drugs 9 (9): 963–9. 2008. PMID 18729003.
- ↑ Oxford Textbook of Medicine: Vol. 1. Oxford University Press. 2003. pp. 1–. ISBN 978-0-19-262922-7. https://books.google.com/books?id=_s65U1n9Lf8C&pg=RA1-PA962.
- ↑ 8.0 8.1 "SC 25152: A potent mineralocorticoid antagonist with reduced affinity for the 5 alpha-dihydrotestosterone receptor of human and rat prostate". J. Clin. Endocrinol. Metab. 47 (1): 171–5. 1978. doi:10.1210/jcem-47-1-171. PMID 263288.
- ↑ "Relationship between covalent binding to microsomal protein and the destruction of adrenal cytochrome P-450 by spironolactone". Toxicology 67 (2): 143–54. April 1991. doi:10.1016/0300-483X(91)90138-Q. PMID 2031249.
- ↑ "Role of the steroid 17 alpha-hydroxylase in spironolactone-mediated destruction of adrenal cytochrome P-450". Mol. Pharmacol. 40 (2): 321–5. August 1991. PMID 1875914.
- ↑ "Oxidative metabolism of spironolactone: evidence for the involvement of electrophilic thiosteroid species in drug-mediated destruction of rat hepatic cytochrome P450". Biochemistry 28 (12): 5128–36. June 1989. doi:10.1021/bi00438a033. PMID 2765527.
- ↑ "Spironolactone metabolism: steady-state serum levels of the sulfur-containing metabolites". J Clin Pharmacol 29 (4): 342–7. 1989. doi:10.1002/j.1552-4604.1989.tb03339.x. PMID 2723123.
- ↑ "Transport of steroid hormones: interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma". J. Clin. Endocrinol. Metab. 53 (1): 69–75. 1981. doi:10.1210/jcem-53-1-69. PMID 7195405.
Further reading
- "Spironolactone metabolism: steady-state serum levels of the sulfur-containing metabolites". J Clin Pharmacol 29 (4): 342–7. 1989. doi:10.1002/j.1552-4604.1989.tb03339.x. PMID 2723123.
 |

