Chemistry:Heterocodeine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Heterocodeine, Morphine 6-methyl ether |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H21NO3 |
| Molar mass | 299.370 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
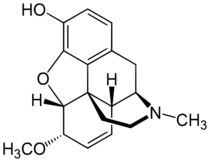
Heterocodeine (6-methoxymorphine) is an opiate derivative, the 6-methyl ether of morphine, and a structural isomer of codeine; it is called "hetero-" because it is the reverse isomer of codeine. Heterocodeine was first synthesised in 1932 and first patented in 1935.[1] It can be made from morphine by selective methylation.[2] Codeine is the natural mono-methyl ether, but must be metabolized for activity (that is, it is a prodrug). In contrast the semi-synthetic mono-methyl ether, heterocodeine is a direct agonist. The 6,7,8,14 tetradehydro 3,6 methyl di-ether of morphine is thebaine.
Heterocodeine is 6 times more potent than morphine[3] due to having a substitution at the 6-hydroxy position, in a similar manner to 6-acetylmorphine.[4] The drug methyldihydromorphine (dihydroheterocodeine) is a derivative of heterocodeine. Like the morphine metabolite morphine-6-glucuronide, 6-position branches (esters or ethers) of morphine bind to the otherwise unagonized human mu receptor subtype mu-3 (or μ3); as well as the 6-acetylmorphine metabolite of heroin this includes heterocodeine.[5]
The relative strength of heterocodeine to codeine has been published as 50, 72, 81, 88, 93, 96, and 108 ×.
It is not mentioned specifically in the Controlled Substances Act 1970 but is a Schedule II controlled substance as an analogue of morphinan or morphine under the morphine structure rules of the Analogues Act; in other countries it is usually controlled as a strong opioid.
Homocodeine is a synonym for pholcodine. Bicodeine is a dimer of codeine which is essentially the codeine analogue of pseudomorphine and is also known as pseudocodeine. It is an occasional component of opium and is also a decomposition product of codeine under certain circumstances.[6]
References
- ↑ US patent 2058521
- ↑ "Synthesis of thebaine and oripavine from codeine and morphine". Journal of Medicinal Chemistry 18 (11): 1074–7. November 1975. doi:10.1021/jm00245a006. PMID 1177252.
- ↑ "Chemistry of Opioid Analgesics". PHA 5155- Neurology Pharmacotherapeutics Medicinal Chemistry Tutorials. http://www.acsmedchem.org/module/opioid.html.
- ↑ "Biological evaluation of compounds for their physical dependence potential & abuse liability. XXIV.". Drug evaluation committee of the college on problems of drug dependence. 2000. http://www.pharmacy.umaryland.edu/faculty/acoop/decfolder/DEC2000.pdf.
- ↑ "3-Methoxynaltrexone, a selective heroin/morphine-6beta-glucuronide antagonist". FEBS Letters 412 (1): 35–8. July 1997. doi:10.1016/S0014-5793(97)00710-2. PMID 9257684.
- ↑ "Status Decision of Controlled and Non-Controlled Substances: Codeine dimer". Health Canada. 15 June 2005. https://isomerdesign.com/Cdsa/HC/StatusDecisions/A-2013-00235%20-%20PDFs/C-Codeine%20dimer%202005-06-16.pdf.
 |
