Chemistry:Taxifolin
| |
| |
| Names | |
|---|---|
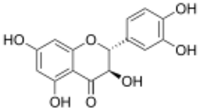
| IUPAC name
(2R,3R)-3,3′,4′,5,7-Pentahydroxyflavan-4-one
| |
| Systematic IUPAC name
(2R,3R)-2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
Dihydroquercetin
Taxifoliol Distylin (+)-Taxifolin trans-Dihydroquercetin (+)-Dihydroquercetin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H12O7 | |
| Molar mass | 304.254 g·mol−1 |
| Appearance | Brown powder |
| Melting point | 237 °C (459 °F; 510 K)[1] |
| UV-vis (λmax) | 290, 327 nm (methanol) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Taxifolin (5,7,3',4'-flavan-on-ol), also known as dihydroquercetin, belongs to the subclass flavanonols in the flavonoids, which in turn is a class of polyphenols. It is extracted from plants such as Siberian larch and milk thistle. [2]
Stereocenters
Taxifolin has two stereocenters on the C-ring, as opposed to quercetin which has none.[3] For example, (+)-taxifolin has (2R,3R)-configuration, making it 1 out of 4 stereoisomers that comprise 2 pairs of enantiomers.[4]
Natural occurrences
Taxifolin is found in non-glutinous rice boiled with adzuki bean (adzuki-meshi).[5]
It can be found in conifers like the Siberian larch, Larix sibirica, in Russia, in Pinus roxburghii,[6] in Cedrus deodara[6] and in the Chinese yew, Taxus chinensis var. mairei.[7]
It is also found in the silymarin extract from the milk thistle seeds.
Taxifolin is present in vinegars aged in cherry wood.[8]
Taxifolin, and flavonoids in general, can be found in many beverages and products. Specifically, taxifolin is found in plant-based foods like fruit, vegetables, wine, tea, and cocoa.[9]
Pharmacology
Taxifolin is not mutagenic and less toxic than the related compound quercetin.[10] It acts as a potential chemopreventive agent by regulating genes via an ARE-dependent mechanism.[11] Taxifolin has shown to inhibit the ovarian cancer cell growth in a dose-dependent manner.[12] However, in this same study, taxifolin was the least effective flavonoid in the inhibition of VEGF expression.[13] There is also a strong correlation (with a correlation coefficient of 0.93) between the antiproliferative effects of taxifolin derivatives on murine skin fibroblasts and human breast cancer cells.[14]
Taxifolin has shown to have anti-proliferative effects on many types of cancer cells by inhibiting cancer cell lipogenesis. By inhibiting the fatty acid synthase in cancer cells, taxifolin is able to prevent the growth and spread of cancer cells.[15]
Taxifolin also stops the effects of overexpression of P-glycoprotein, which prevents the development of chemoresistance. Taxifolin does this via inhibition of rhodamine 123 and doxorubicin.[16]
The capacity of taxifolin to stimulate fibril formation and promote stabilization of fibrillar forms of collagen can be used in medicine.[17] Also taxifolin inhibited the cellular melanogenesis as effectively as arbutin, one of the most widely used hypopigmenting agents in cosmetics.
Taxifolin also enhances the efficacy of conventional antibiotics like levofloxacin and ceftazidime in vitro, which have potential for combinatory therapy of patients infected with methicillin-resistant Staphylococcus aureus (MRSA).[18]
Taxifolin can act as an anti-flammant because of its ability to inhibit the synthesis of cyclooxygenase by blocking prostaglandin synthesis. [19] Indeed, the taxifolin-mediated inhibition of the E2 prostaglandin synthesis (by PLA2 phospholipase) was shown to prevent beta-amyloid-induced impairment of synapsis genesis and related memory deficits, which take part in the pathogenesis of neurodegenerative disorders like Alzheimer's disease.[20] Other observed benefits comprise the reduction of beta-amyloid accumulation in the brain vessels, restoration of vascular integrity and memory improvement in cerebral amyloid angiopathy, a condition often linked to alzheimer's.[21] Cognitive performance has even been raised in young healthy adults by taxifolin administration.[22]
Like other flavonoids, taxifolin is able to function as an antifungal agent by blocking multiple pathways that promote the growth and proliferation of fungi. [19]
Taxifolin has also been found to reduce inhibitor of intestinal mobility especially when antagonized by verapamil.[19]
Taxifolin has also been shown to be anti-hyperlipidemic by maintaining the normal lipid profile of the liver and keeping lipid excretion at normal levels. Taxifolin prevents hyperlipidemia by reducing the esterification of cellular cholesterol, phospholipid, and triacylglycerol synthesis.[16]
Taxifolin, as well as many other flavonoids, has been found to act as a non-selective antagonist of the opioid receptors, albeit with somewhat weak affinity.[23] Taxifolin shows promising pharmacological activities in the management of inflammation, tumors, microbial infections, oxidative stress, cardiovascular, and liver disorders [24]
Taxifolin has been found to act as an agonist of the adiponectin receptor 2 (AdipoR2).[25]
Metabolism
The enzyme taxifolin 8-monooxygenase uses taxifolin, NADH, NADPH, H+, and O2 to produce 2,3-dihydrogossypetin, NAD+, NADP+, and H2O.
The enzyme leucocyanidin oxygenase uses leucocyanidin, alpha-ketoglutarate, and O2 to produce cis-dihydroquercetin, taxifolin, succinate, CO2, and H2O.
Glycosides
Astilbin is the 3-O-rhamnoside of taxifolin. Taxifolin deoxyhexose can be found in açai fruits.[26]
Taxifolin 3-O-glucoside isomers have been separated from Chamaecyparis obtusa.[27]
(-)-2,3-trans-Dihydroquercetin-3'-O-β-D-glucopyranoside, a taxifolin glucoside has been extracted from the inner bark of Pinus densiflora and can act as an oviposition stimulant in the cerambycid beetle Monochamus alternatus.[28]
(2S,3S)-(-)-Taxifolin-3-O-β-D-glucopyranoside has been isolated from the root-sprouts of Agrimonia pilosa.[29]
(2R,3R)-Taxifolin-3'-O-β-D-pyranoglucoside has been isolated from the rhizome of Smilax glabra.[30]
Minor amount of taxifolin 4′-O-β-glucopyranoiside can be found in red onions.[31]
(2R,3R)-Taxifolin 3-O-arabinoside and (2S,3S)-taxifolin 3-O-arabinoside have been isolated from the leaves of Trachelospermum jasminoides[32] (star jasmine).
Derived natural compounds
- Dihydroquercetin-3-O-rhamnoside (Astilbin)
- (+)-Leucocyanidin can be synthesized from taxifolin by sodium borohydride reduction.[33]
References
- ↑ Graham, H. M.; Kurth, E. F. (1949). "Constituents of Extractives from Douglas Fir". Industrial and Engineering Chemistry 41 (2): 409–414. doi:10.1021/ie50470a035.
- ↑ Title = Global Taxifolin Market Size was USD 167.90 Million in 2022| URL = https://www.reportprime.com/taxifolin-r195 | Date = 14 August 2023 | Website = https://www.reportprime.com/
- ↑ "Quercetin". https://pubchem.ncbi.nlm.nih.gov/compound/5280343.
- ↑ "(+)-taxifolin (CHEBI:17948)". https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:17948.
- ↑ Takahama (2016-10-01). "Antioxidative flavonoids in adzuki-meshi (rice boiled with adzuki bean) react with nitrite under simulated stomach conditions". Journal of Functional Foods 26: 657–666. doi:10.1016/j.jff.2016.08.032. https://www.sciencedirect.com/science/article/abs/pii/S1756464616302468.
- ↑ 6.0 6.1 "Extractives in bark of different conifer species growing in Pakistan". Holzforschung 63 (5): 551–558. August 2009. doi:10.1515/HF.2009.095.
- ↑ Li, Cunfang (2008). "Chemistry of Chinese yew, Taxus chinensis var. mairei". Biochemical Systematics and Ecology 36 (4): 266–282. doi:10.1016/j.bse.2007.08.002.
- ↑ Cerezoa, Ana B.; Tesfayea, Wendu; Soria-Díazb, M.E.; Torijac, M. Jesús; Mateoc, Estíbaliz; Garcia-Parrillaa, M. Carmen; Troncosoa, Ana M. (2010). "Effect of wood on the phenolic profile and sensory properties of wine vinegars during ageing". Journal of Food Composition and Analysis 23 (2): 175–184. doi:10.1016/j.jfca.2009.08.008.
- ↑ Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J. (2005). "Induction of Cancer Cell Apoptosis by Flavonoids Is Associated with Their Ability to Inhibit Fatty Acid Synthase Activity". Journal of Biological Chemistry 280 (7): 5636–5645. doi:10.1074/jbc.m408177200. PMID 15533929.
- ↑ Makena, Patrudu S.; Pierce, Samuel C.; Chung, King-Thom; Sinclair, Scott E. (2009). "Comparative mutagenic effects of structurally similar flavonoids quercetin and taxifolin on tester strains Salmonella typhimurium TA102 and Escherichia coli WP-2 uvrA". Environmental and Molecular Mutagenesis 50 (6): 451–9. doi:10.1002/em.20487. PMID 19326464.
- ↑ Lee, Saet Byoul; Cha, Kwang Hyun; Selenge, Dangaa; Solongo, Amgalan; Nho, Chu Won (2007). "The Chemopreventive Effect of Taxifolin Is Exerted through ARE-Dependent Gene Regulation". Biological & Pharmaceutical Bulletin 30 (6): 1074–9. doi:10.1248/bpb.30.1074. PMID 17541156.
- ↑ Luo, Haitao; Jiang, Bing-Hua; King, Sarah; Chen, Yi Charlie (2008). "Inhibition of Cell Growth and VEGF Expression in Ovarian Cancer Cells by Flavonoids". Nutrition and Cancer 60 (6): 800–9. doi:10.1080/01635580802100851. PMID 19005980.
- ↑ Luo, Haitao; Jiang, Bing-Hua; King, Sarah M.; Chen, Yi Charlie (2008). "Inhibition of Cell Growth and VEGF Expression in Ovarian Cancer Cells by Flavonoids". Nutrition and Cancer 60 (6): 800–809. doi:10.1080/01635580802100851. PMID 19005980.
- ↑ "[Antiproliferative and antioxidant activity of new dihydroquercetin derivatives]" (in ru). Eksp Klin Farmakol 73 (9): 39–42. 2010. PMID 21086652.
- ↑ Brusselmans, Koen; Vrolix, Ruth; Verhoeven, Guido; Swinnen, Johannes V. (2005). "Induction of Cancer Cell Apoptosis by Flavonoids is Associated with Their Ability to Inhibit Fatty Acid Synthase Activity". Journal of Biological Chemistry 280 (7): 5636–5645. doi:10.1074/jbc.M408177200. PMID 15533929.
- ↑ 16.0 16.1 Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A. K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomedicine & Pharmacotherapy 2021, 142, 17.
- ↑ Tarahovsky, Y. S.; Selezneva, I. I.; Vasilieva, N. A.; Egorochkin, M. A.; Kim, Yu. A. (2007). "Acceleration of fibril formation and thermal stabilization of collagen fibrils in the presence of taxifolin (dihydroquercetin)". Bulletin of Experimental Biology and Medicine 144 (6): 791–4. doi:10.1007/s10517-007-0433-z. PMID 18856203.
- ↑ "Antibacterial and synergy of a flavanonol rhamnoside with antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA)". Phytomedicine 18 (11): 990–3. August 2011. doi:10.1016/j.phymed.2011.02.013. PMID 21466953.
- ↑ 19.0 19.1 19.2 Saftar Asmi, K., et al. “Therapeutic Aspects of Taxifolin – An Update.” Therapeutic Aspects of Taxifolin – An Update - Journal of Advanced Pharmacy Education and Research, SPER Publications and Solutions Pvt. Ltd., 2017, https://japer.in/article/therapeutic-aspects-of-taxifolin-an-update .
- ↑ Wang (2018). Taxifolin.
- ↑ Saito, 2017
- ↑ Shinozaki, 2023
- ↑ "Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships". J. Nat. Prod. 70 (8): 1278–82. August 2007. doi:10.1021/np070194x. PMID 17685652.
- ↑ Sunil, Christudas; Xu, Baojun (2019). "An insight into the health-promoting effects of taxifolin (dihydroquercetin)". Phytochemistry 166: 112066. doi:10.1016/J.PHYTOCHEM.2019.112066. PMID 31325613.
- ↑ "Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay". PLOS ONE 8 (5): e63354. 2013. doi:10.1371/journal.pone.0063354. PMID 23691032. Bibcode: 2013PLoSO...863354S.
- ↑ Gallori, S. (2004). "Polyphenolic Constituents of Fruit Pulp of Euterpe oleracea Mart. (Açai palm)". Chromatographia 59 (11–12). doi:10.1365/s10337-004-0305-x.
- ↑ Sakushima, Akiyo; Ohno, Kosei; Coskun, Makusut; Seki, Koh-Ichi; Ohkura, Kazue (2002). "Separation and Identification of Taxifolin 3- O -Glucoside Isomers from Chamaecyparis Obtusa (Cupressaceae)". Natural Product Research 16 (6): 383–7. doi:10.1080/10575630290033141. PMID 12462342.
- ↑ Sato, Masashi; Islam, Syed Q.; Awata, Shinobu; Yamasaki, Tory (1999). "Flavanonol glucoside and proanthocyanidins: Oviposition stimulants for the cerambycid beetle, Monochamus alternatus". Journal of Pesticide Science 24 (2): 123–9. doi:10.1584/jpestics.24.123.
- ↑ "[Studies on the structure of a new flavanonol glucoside of the root-sprouts of Agrimonia pilosa Ledeb]" (in zh). Yao Xue Xue Bao 25 (4): 267–70. 1990. PMID 2281787.
- ↑ "[Studies on dihydroflavonol glycosides from rhizome of Smilax glabra]" (in zh). Zhongguo Zhong Yao Za Zhi 29 (9): 867–70. September 2004. PMID 15575206.
- ↑ Fossen, Torgils (1998). "Flavonoids from red onion (Allium cepa)". Phytochemistry 47 (2): 281–285. doi:10.1016/S0031-9422(97)00423-8.
- ↑ Hosoi, Shinzo; Shimizu, Eri; Ohno, Kosei; Yokosawa, Ryozo; Kuninaga, Shiro; Coskun, Maksut; Sakushima, Akiyo (2006). "Structural Studies of Zoospore Attractants from Trachelospermum jasminoides var. pubescens: Taxifolin 3-O-glycosides". Phytochemical Analysis 17 (1): 20–4. doi:10.1002/pca.876. PMID 16454472.
- ↑ Heller, Werner; Britsch, Lothar; Forkmann, Gert; Grisebach, Hans (1985). "Leucoanthocyanidins as intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana R. Br". Planta 163 (2): 191–6. doi:10.1007/BF00393505. PMID 24249337.
 |

