Chemistry:Zonisamide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zonegran, Zonisade |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603008 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100%[3] |
| Protein binding | 40%[3] |
| Metabolism | Liver through CYP3A4[3] |
| Elimination half-life | 63 hours in plasma[3] |
| Excretion | Kidney (62%); Faeces (3%)[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
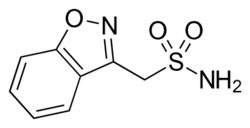
| Formula | C8H8N2O3S |
| Molar mass | 212.22 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 162 °C (324 °F) |
| |
| |
| (verify) | |
Zonisamide, sold under the brand name Zonegran among others, is a medication used to treat the symptoms of epilepsy and Parkinson's disease.[4][5] Chemically it is a sulfonamide. It serves as an anticonvulsant used primarily as an adjunctive therapy in adults with Parkinson's disease, partial-onset seizures; infantile spasm, mixed seizure types of Lennox–Gastaut syndrome, myoclonic and generalized tonic clonic seizure.[6] Despite this it is also sometimes used as a monotherapy for partial-onset seizures.[5][7]
In 2020, it was the 276th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[8][9]
Medical uses
Epilepsy
Zonisamide is approved in the United States,[1][10] and United Kingdom[11] for adjunctive treatment of partial seizures in adults and Japan for both adjunctive and monotherapy for partial seizures (simple, complex, secondarily generalized), generalized (tonic, tonic-clonic (grand mal), and atypical absence) and combined seizures.[12] In Australia it is marketed as both an adjunctive therapy and monotherapy for partial seizures only.[7]
Parkinson's disease
It has been approved for the treatment of the motor symptoms of Parkinson's disease (PD), as an adjunct to levodopa, in a few countries such as Japan.[4][5] In Japan, zonisamide has been used as an adjunct to levodopa treatment since 2009.[13] In addition, there is clinical evidence that zonisamide in combination with levodopa control of motor symptoms of PD but evidence for the treatment of the non motor symptoms of PD lacking.[14][15]
Adverse effects
Adverse effects by incidence:[3][16][17]
Very common (>10% incidence) adverse effects include:
- Anorexia
- Somnolence
- Dizziness
- Agitation
- Irritability
- Confusional state
- Depression
- Diplopia
- Memory impairment
- Decreased bicarbonate
Common (1-10% incidence) adverse effects include:
- Ecchymosis
- Hypersensitivity
- Affect lability
- Anxiety
- Insomnia
- Psychotic disorder
- Bradyphrenia
- Disturbance in attention
- Nystagmus
- Paraesthesia
- Speech disorder
- Tremor
- Abdominal pain
- Constipation
- Diarrhoea
- Dyspepsia
- Nausea
- Rash
- Pruritus
- Alopecia
- Nephrolithiasis
- Fatigue
- Influenza-like illness
- Pyrexia
- Oedema peripheral
- Weight loss
Interactions
Zonisamide and other carbonic anhydrase inhibitors such as topiramate, furosemide, and hydrochlorothiazide have been known to interfere with amobarbital, which has led to inadequate anesthetization during the Wada test.[18] Zonisamide may also interact with other carbonic anhydrase inhibitors to increase the potential for metabolic acidosis.[3]
Additionally, the metabolism of zonisamide is inhibited by ketoconazole, ciclosporin, miconazole, fluconazole and carbamazepine (in descending order of inhibition) due to their effects on the CYP3A4 enzyme.[19]
Zonisamide is not known to inhibit cytochrome P450 enzymes when present at therapeutic concentrations.[20]
Mechanism of action
Zonisamide is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. The precise mechanism by which zonisamide exerts its antiseizure effect is unknown, although it is believed that the drug blocks sodium and T-type calcium channels, which leads to the suppression of neuronal hypersynchronization (that is, seizure-form activity).[7] It is also known to be a weak carbonic anhydrase inhibitor (similarly to the anticonvulsant topiramate). It is also known to modulate GABAergic and glutamatergic neurotransmission.[7][21][22][23][24]
Pharmacokinetics
Absorption
Variable, yet relatively rapid rate of absorption with a time to peak concentration of 2.8-3.9 hours. Bioavailability is close to 100% and food has no effect on the bioavailability of zonisamide but may affect the rate of absorption.[25][20]
Metabolism
Zonisamide is metabolized mostly by the CYP3A4 isoenzyme, but also CYP3A7 and CYP3A5,[26] to 2-(sulphamoylacetyl)-phenol via reductive cleavage of the 1,2-benzisoxazole ring.[27]
History
Zonisamide was discovered by Uno and colleagues in 1972[28] and launched by Dainippon Sumitomo Pharma (formerly Dainippon Pharmaceutical) in 1989 as Excegran in Japan.[29] It was marketed by Élan in the United States starting in 2000 as Zonegran, before Élan transferred their interests in zonisamide to Eisai Co., Ltd. in 2004.[30] Eisai also markets Zonegran in Asia (China, Taiwan, and fourteen others)[31] and Europe (starting in Germany and the United Kingdom).[32]
Research
Tardive dyskinesia
In an open-label trial zonisamide attenuated the symptoms of tardive dyskinesia.[33]
Obesity
It has also been studied for obesity[34] with significant positive effects on body weight loss and there are three ongoing clinical trials for this indication.[35][36][37] It was to be sold, when combined with bupropion, under the brand name Empatic, until its development was discontinued.[38]
Migraine
Zonisamide has been studied for and used as a migraine preventative medication, when topiramate is either ineffective or cannot be continued due to side effects.[5]
Bipolar depression
It has also been used off-label by psychiatrists as a mood stabilizer to treat bipolar depression.[39][40]
References
- ↑ 1.0 1.1 "Zonegran- zonisamide capsule". 20 August 2021. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d12de43e-3ac3-4335-bc85-70d7366a91eb.
- ↑ "Zonisade- zonisamide suspension". 15 July 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac16fa15-32e9-4f92-8bc6-d8d41ae002c6.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Zonegran Product Information" (PDF). TGA eBusiness Services. SciGen (Australia) Pty Ltd. 4 April 2013. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2011-PI-02002-3.
- ↑ 4.0 4.1 "Zonisamide: a review of the clinical and experimental evidence for its use in Parkinson's disease". Indian Journal of Pharmacology 45 (6): 547–55. November–December 2013. doi:10.4103/0253-7613.121266. PMID 24347760.
- ↑ 5.0 5.1 5.2 5.3 Brayfield, A, ed (8 March 2016). "Zonisamide: Martindale: The Complete Drug Reference". London, UK: Pharmaceutical Press. https://www.medicinescomplete.com/mc/martindale/current/1668-x.htm.
- ↑ Comprehensive Pharmacy Review (6th ed.). Williams & Wilkins. 2007. p. 988. ISBN 9780781765619. OCLC 869677890.
- ↑ 7.0 7.1 7.2 7.3 Rossi, S, ed (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Zonisamide - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Zonisamide.
- ↑ "Drug Approval Package: Zonegran (Zonisomide) NDA #20-789". 24 December 1999. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/020789_Zonegran.cfm.
- ↑ Eisai Ltd. (2005). "Zonegran Summary of Product Characteristics". electronic Medicines Compendium. Medicines.org.uk. http://emc.medicines.org.uk/.
- ↑ Dainippon Pharmaceutical Co., Ltd. (2004). "EXCEGRAN Tablets 100 mg & EXCEGRAN Powder 20%". http://www.e-search.ne.jp/~jpr/PDF/DAINIP03.PDF.
- ↑ "Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study". Neurology 68 (1): 45–50. January 2007. doi:10.1212/01.wnl.0000250236.75053.16. PMID 17200492.
- ↑ "Zonisamide: a review of the clinical and experimental evidence for its use in Parkinson's disease". Indian Journal of Pharmacology 45 (6): 547–55. 2013. doi:10.4103/0253-7613.121266. PMID 24347760.
- ↑ "Combination Therapy with Zonisamide and Antiparkinson Drugs for Parkinson's Disease: A Meta-Analysis". Journal of Alzheimer's Disease 56 (4): 1229–1239. 2017. doi:10.3233/JAD-161068. PMID 28157097.
- ↑ "Zonegran 25, 50, 100 mg Hard Capsules". electronic Medicines Compendium. Eisai Ltd. 8 October 2013. http://www.medicines.org.uk/emc/medicine/16240/SPC/Zonegran+25%2c+50%2c+100+mg+Hard+Capsules/.
- ↑ "zonisamide (Rx) - Zonegran". Medscape Reference. WebMD. http://reference.medscape.com/drug/zonegran-zonisamide-343025.
- ↑ "Reduced anesthetization during the intracarotid amobarbital (Wada) test in patients taking carbonic anhydrase-inhibiting medications". Epilepsia 46 (2): 236–43. February 2005. doi:10.1111/j.0013-9580.2005.23904.x. PMID 15679504.
- ↑ "Prediction of drug-drug interactions of zonisamide metabolism in humans from in vitro data". European Journal of Clinical Pharmacology 54 (2): 177–83. April 1998. doi:10.1007/s002280050442. PMID 9626925.
- ↑ 20.0 20.1 "Zonegran 25, 50, 100 mg Hard Capsules". Electronic Medicines Compendium (eMC). https://www.medicines.org.uk/emc/medicine/16240.
- ↑ "Zonisamide: chemistry, mechanism of action, and pharmacokinetics". Seizure 13 (Suppl 1): S5–9; discussion S10. December 2004. doi:10.1016/j.seizure.2004.04.016. PMID 15511691.
- ↑ "Interaction of zonisamide with benzodiazepine and GABA receptors in rat brain". Medical Journal of Osaka University 39 (1–4): 13–7. March 1990. PMID 1369646.
- ↑ "[3H]zonisamide binding in rat brain". Medical Journal of Osaka University 39 (1–4): 19–22. March 1990. PMID 1369647.
- ↑ "Effect of zonisamide on molecular regulation of glutamate and GABA transporter proteins during epileptogenesis in rats with hippocampal seizures". Brain Research. Molecular Brain Research 116 (1–2): 1–6. August 2003. doi:10.1016/S0169-328X(03)00183-9. PMID 12941455.
- ↑ "Zonisamide". https://www.drugbank.ca/drugs/DB00909.
- ↑ "Differential catalytic properties in metabolism of endogenous and exogenous substrates among CYP3A enzymes expressed in COS-7 cells". Biochimica et Biophysica Acta (BBA) - General Subjects 1380 (3): 297–304. May 1998. doi:10.1016/s0304-4165(97)00156-6. PMID 9555064.
- ↑ "Reductive metabolism of the anticonvulsant agent zonisamide, a 1,2-benzisoxazole derivative". Xenobiotica 22 (1): 1–11. January 1992. doi:10.3109/00498259209053097. PMID 1615700.
- ↑ "Zonisamide". Antiepileptic Drugs (Fifth ed.). Philadelphia: Lippincott Williams & Wilkins. 2002-06-15. p. 873. ISBN 0-7817-2321-3. https://books.google.com/books?id=HAOY0qG-vAYC&q=zonisamide+synthesized&pg=PA873. Retrieved 2007-11-07.
- ↑ Dainippon Sumitomo Pharma Co. Ltd. (2005). "Company History". Company Information. Dainippon Sumitomo Co., Ltd.. http://www.ds-pharma.co.jp/english/profile/history.html.
- ↑ Dainippon Pharmaceutical Co. Ltd. (2004). "Transfer of Rights Agreement for North America and Europe Reached on Zonegran". News Releases for Dainippon Pharmaceutical in 2004. Dainippon Sumitomo Pharma Co., Ltd. http://www.ds-pharma.co.jp/english/news/dainippon_2004.html.
- ↑ Dainippon Pharmaceutical Co. Ltd. (2005). "Dainippon Pharmaceutical and Eisai Conclude Agreement for the Development, Manufacture and Marketing of the Anti-Epileptic Agent Zonisamide in Asia". Dainippon Pharmaceutical News Releases for 2005. Dainippon Sumitomo Pharma Co., Ltd.. http://www.ds-pharma.co.jp/english/news/dainippon_2005/no_002.html.
- ↑ Eisai Co. Ltd. (2005). "Eisai Announces Launch of Zonegran (zonisamide), Treatment For Epilepsy In the UK and Germany". Eisai 2005 News Releases. Eisai Co., Ltd.. http://www.eisai.co.jp/enews/index.html.
- ↑ "Effects of zonisamide on tardive dyskinesia: a preliminary open-label trial". Journal of the Neurological Sciences 315 (1–2): 137–40. April 2012. doi:10.1016/j.jns.2011.12.010. PMID 22285275.
- ↑ "Zonisamide for weight loss in obese adults: a randomized controlled trial". JAMA 289 (14): 1820–5. April 2003. doi:10.1001/jama.289.14.1820. PMID 12684361.
- ↑ University of Cincinnati (2005). "Zonegran in the Treatment of Binge Eating Disorder Associated With Obesity". ClinicalTrials.gov. http://clinicaltrials.gov/show/NCT00221442.
- ↑ "Zonegran for the Treatment of Weight Gain Associated With Psychotropic Medication Use: A Placebo-Controlled Trial". ClinicalTrials.gov. 2005. http://clinicaltrials.gov/show/NCT00203450.
- ↑ National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (2006). "Zonisamide for Weight Reduction in Obese Adults". ClinicalTrials.gov. http://clinicaltrials.gov/show/NCT00275834.
- ↑ "Bupropion/zonisamide". Springer. 20 May 2017. http://adisinsight.springer.com/drugs/800024638.
- ↑ Loftus, Brian D. (2004). "Zonegran". http://www.bellaireneurology.com/seizure/epil_trt_zonegran.html.
- ↑ "Utilization of zonisamide in patients with chronic pain or epilepsy refractory to other treatments: a retrospective, open label, uncontrolled study in a VA hospital". Current Medical Research and Opinion 20 (5): 577–80. May 2004. doi:10.1185/030079904125003313. PMID 15140322.
External links
- "Zonisamide". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/zonisamide.
 |

