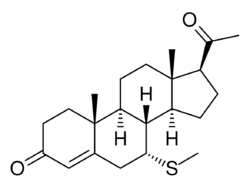
Chemistry:7α-Thioprogesterone
 | |
| Clinical data | |
|---|---|
| Other names | 7α-TP4; SC-8365; 7α-Mercaptopregn-4-ene-3,20-dione |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C21H28O2S |
| Molar mass | 344.51 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
7α-Thioprogesterone (7α-TP4; developmental code name SC-8365; also known as 7α-mercaptopregn-4-ene-3,20-dione) is a synthetic, steroidal, and potent antimineralocorticoid (putative) and antiandrogen which was developed by G. D. Searle & Co and was described in the late 1970s and early 1980s but was never developed or introduced for medical use.[1][2][3] It is a derivative of progesterone (pregn-4-ene-3,20-dione) with a thio (sulfur) substitution at the C7α position, and is related to the spirolactone group of drugs but lacks a γ-lactone ring.[1][2]
As an antiandrogen, 7α-TP4 has approximately 8.5% of the affinity of dihydrotestosterone (DHT) for the rat ventral prostate androgen receptor (AR), which is similar to that of spironolactone and its active metabolite 7α-thiomethylspironolactone.[1] The drug has also been assessed at steroid hormone-associated carrier proteins, and shows very low binding to sex hormone-binding globulin (SHBG) but high affinity for corticosteroid-binding globulin (CBG) approximately equal to that of progesterone.[2]
7α-Acetylthio-17α-hydroxyprogesterone, a related derivative of progesterone and also of 17α-hydroxyprogesterone, has been found to possess potent antimineralocorticoid activity similarly.[4] Spironolactone is the derivative of this compound in which the acetyl group at the C17β position has been cyclized with the C17α hydroxyl group to form a spiro 21-carboxylic acid γ-lactone ring.[5][6][7]
References
- ↑ 1.0 1.1 1.2 "SC 25152: A potent mineralocorticoid antagonist with reduced affinity for the 5 alpha-dihydrotestosterone receptor of human and rat prostate". J. Clin. Endocrinol. Metab. 47 (1): 171–5. 1978. doi:10.1210/jcem-47-1-171. PMID 263288.
- ↑ 2.0 2.1 2.2 "Transport of steroid hormones: interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma". J. Clin. Endocrinol. Metab. 53 (1): 69–75. 1981. doi:10.1210/jcem-53-1-69. PMID 7195405.
- ↑ Ulrich Westphal (6 December 2012). Steroid-Protein Interactions II. Springer Science & Business Media. pp. 501–. ISBN 978-3-642-82486-9. https://books.google.com/books?id=3DD6CAAAQBAJ&pg=PA501.
- ↑ Ralph I. Dorfman (5 December 2016). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 395–. ISBN 978-1-4832-7299-3. https://books.google.com/books?id=BbLfBAAAQBAJ&pg=PA395.
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 1111. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA1111.
- ↑ "Spironolactone". https://pubchem.ncbi.nlm.nih.gov/compound/5833.
- ↑ "Spironolactone | C24H32O4S | ChemSpider". http://www.chemspider.com/Chemical-Structure.5628.html?rid=b578a389-d64b-4ec2-ae20-309e5f3abd11.
 |

