Chemistry:Bromoketamine
From HandWiki
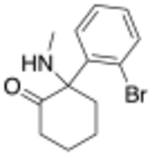
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-(2-Bromophenyl)-2-(methylamino)cyclohexan-1-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C13H16BrNO | |
| Molar mass | 282.181 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Bromoketamine or 2-bromodeschloroketamine is a chemical compound of the arylcyclohexylamine class, which is an analog of the dissociative anesthetic drug ketamine in which the chlorine atom has been replaced with a bromine atom. It is used in scientific research as a comparison or control compound in studies into the metabolism of ketamine and norketamine,[1][2][3] and has also been sold online alongside arylcyclohexylamine designer drugs, though it is unclear whether bromoketamine has similar pharmacological activity.[citation needed]
See also
- 2-Fluorodeschloroketamine
- 3-Fluorodeschloroketamine
- Deschloroketamine
- Methoxyketamine
- Trifluoromethyldeschloroketamine
References
- ↑ "Gas chromatographic analysis of ketamine and norketamine in plasma and urine: nitrogen-sensitive detection". Journal of Chromatography 232 (2): 305–14. November 1982. doi:10.1016/s0378-4347(00)84170-5. PMID 6818238.
- ↑ "Determination of the enantiomers of ketamine and norketamine in human plasma by enantioselective liquid chromatography-mass spectrometry". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 794 (1): 99–108. August 2003. doi:10.1016/s1570-0232(03)00420-3. PMID 12888202.
- ↑ "Halogen Substitution Influences Ketamine Metabolism by Cytochrome P450 2B6: In Vitro and Computational Approaches". Molecular Pharmaceutics 16 (2): 898–906. February 2019. doi:10.1021/acs.molpharmaceut.8b01214. PMID 30589555.

