Chemistry:Tizanidine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /taɪˈzænɪdiːn/ tye-ZAN-i-deen |
| Trade names | Zanaflex, Sirdalud, and others |
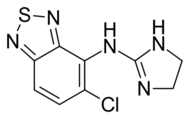
| Other names | 4-Chloro-N-(4,5-dihydro-1H-imidazol-2-yl)-8-thia-7,9-diazabicyclo[4.3.0]nona-2,4,6,9-tetraen-5-amine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601121 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | α2-adrenergic receptor agonist |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~40%[1] |
| Protein binding | ~30% |
| Metabolism | Liver (CYP1A2, 95%) |
| Elimination half-life | 2.54 hours (tizanidine), 20–40 hours (inactive metabolites)[1] |
| Excretion | Urine (60%), feces (20%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C9H8ClN5S |
| Molar mass | 253.71 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tizanidine, sold under the brand name Zanaflex among others, is an alpha-2 (α2) adrenergic receptor agonist,[2] similar to clonidine, that is used to treat muscle spasticity due to spinal cord injury, multiple sclerosis, and spastic cerebral palsy.[3] Effectiveness appears similar to baclofen or diazepam.[4] It is taken by mouth.[5]
Common side effects of tizanidine include dry mouth, sleepiness, weakness, and dizziness.[5] Serious side effects may include low blood pressure, liver problems, psychosis, and QT prolongation.[5] It is unclear if use in pregnancy and breastfeeding is safe.[6] It is an α2-adrenergic agonist, but how it works is not entirely clear.[5]
Tizanidine was approved for medical use in the United States in 1996.[5] It is available as a generic medication.[3] In 2020, it was the 84th most commonly prescribed medication in the United States, with more than 8 million prescriptions.[7][8]
Medical uses
Tizanidine has been found to be as effective as other antispasmodic drugs and is more tolerable than baclofen and diazepam.[4]
Side effects
Side effects include dizziness, drowsiness, weakness, nervousness, confusion, hallucinations, strange dreams, depression, vomiting, dry mouth, constipation, diarrhea, stomach pain, heartburn, increased muscle spasms, back pain, rash, sweating, and a tingling sensation in the arms, legs, hands, and feet.[9]
Symptoms of overdose in 45 cases reported to a poison control center included: lethargy, bradycardia, hypotension, agitation, confusion, vomiting and coma.[10]
Interactions
Concomitant use of tizanidine and moderate or potent CYP1A2 inhibitors (such as zileuton, certain antiarrhythmics (amiodarone, mexiletine, propafenone, verapamil), cimetidine, famotidine, aciclovir, ticlopidine and oral contraceptives) is contraindicated. Concomitant use of tizanidine with fluvoxamine, a potent CYP1A2 inhibitor in humans, resulted in a 33-fold increase in the tizanidine AUC (plasma drug concentration-time curve).[1] For this reason both fluvoxamine and tizanidine should not be taken at the same time. Fluoroquinolone antibiotics such as moxifloxacin, levofloxacin, and ciprofloxacin should also be avoided due to an increased serum concentration of tizanidine when administered concomitantly.[11] Tizanidine has the potential to interact with other central nervous system depressants. Alcohol should be avoided, particularly as it can upset the stomach. The CNS-depressant effects of tizanidine and alcohol are additive.[1] Caution with the following interactions:[12][13][14]
- antibiotics such as enoxacin, gatifloxacin, levofloxacin, lomefloxacin, moxifloxacin, ofloxacin, sparfloxacin, trovafloxacin, or norfloxacin;
- blood pressure medications such as clonidine, guanabenz, guanfacine (Tenex), or methyldopa;
- heart rhythm medications such as amiodarone (Cordarone, Pacerone), mexiletine (Mexitil), propafenone (Rhythmol), and verapamil (Calan, Covera, Isoptin).
Pharmacology
Tizanidine is an α2 receptor agonist closely related to clonidine. It has approximately one tenth to one fifteenth of the blood pressure lowering effect of clonidine. The relation between the α2 receptor agonism and the spasmolytic action is still not fully understood.[2]
| Site | Ki (nM) | Species | Ref |
|---|---|---|---|
| α2A | 62 | Human | [15] |
| α2B | 75 | OK | [15] |
| α2C | 76 | Rat | [15] |
Route of administration
Tizanidine is available as a tablet or capsule. Capsules may be opened and sprinkled on food. However, this may change the absorption of the medication compared to taking the capsule whole.[16] It has a volume of distribution of 2.4 L/kg following intravenous administration.[1]
Chemistry
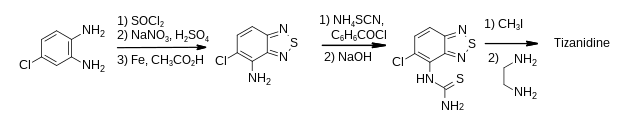
Tizanidine is a derivative of 2,1,3-benzothiadiazole and its first published synthesis was reported in a patent.[17] The 5-chloro-2,1,3-benzothiadiazol-4-amine intermediate was a known compound, produced in three steps from 4-chlorophenylenediamine as shown.[18] Treatment with two equivalents of thionyl chloride in pyridine formed the heterocycle, which was nitrated with sodium nitrate in sulfuric acid and reduced using iron and acetic acid.
The amine intermediate was treated with benzoyl chloride and ammonium thiocyanate followed by alkaline hydrolysis to form a thiourea. This was activated as its isothiuronium salt before being treated with ethylene diamine to give tizanidine.[17][19]
See also
- Xylazine, an alpha-2 (α2) adrenergic receptor agonist and clonidine analogue used in veterinary medicine and as a street drug
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Zanaflex (tizanidine hydrochloride) Capsules and Tablets for Oral Use. Full Prescribing Information". Acorda Therapeutics Inc. Ardsley, NY 10502. http://www.acorda.com/assets/docs/zanaflexpi-final-fda-approved-subm111813.pdf.
- ↑ 2.0 2.1 Basic & clinical pharmacology (14th ed.). New York: McGraw Hill Education. 30 November 2017. p. 487. ISBN 9781259641152. OCLC 1015240036.
- ↑ 3.0 3.1 British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 1094. ISBN 9780857113382.
- ↑ 4.0 4.1 "A practical overview of tizanidine use for spasticity secondary to multiple sclerosis, stroke, and spinal cord injury". Current Medical Research and Opinion 24 (2): 425–439. February 2008. doi:10.1185/030079908X261113. PMID 18167175.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Tizanidine Hydrochloride Monograph for Professionals" (in en). American Society of Health-System Pharmacists. https://www.drugs.com/monograph/tizanidine-hydrochloride.html.
- ↑ "Tizanidine Pregnancy and Breastfeeding Warnings" (in en). https://www.drugs.com/pregnancy/tizanidine.html.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Tizanidine - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Tizanidine.
- ↑ "Page not available". https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0000106/.
- ↑ "Retrospective review of Tizanidine (Zanaflex) overdose". Journal of Toxicology. Clinical Toxicology 42 (5): 593–596. January 2004. doi:10.1081/CLT-200026978. PMID 15462150.
- ↑ "Tizanidine Uses, Dosage, Side Effects – Drugs.com". https://www.drugs.com/tizanidine.html.
- ↑ NHS Wales (2011). "Tizanidine (Zanaflex) Gwent Primary Care Prescribing Guidance". Aneurin Bevan University Health Board. http://www.wales.nhs.uk/sites3/Documents/814/TIZANIDINE-GwentGuidelines%5BJan08%5D.pdf.
- ↑ "Tizanidine package leaflet: Information for the user". Medicines and Healthcare products Regulatory Agency (UK). 2016. http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1496381982517.pdf.
- ↑ "Zanaflex (tizanidine hydrochloride) dose, indications, adverse effects, interactions... from PDR.net" (in en). http://www.pdr.net/drug-summary/Zanaflex-tizanidine-hydrochloride-811.
- ↑ 15.0 15.1 15.2 "Characterization of the alpha-2C adrenergic receptor subtype in the opossum kidney and in the OK cell line". The Journal of Pharmacology and Experimental Therapeutics 259 (1): 323–329. October 1991. PMID 1656026.
- ↑ "Tizanidine: MedlinePlus Drug Information" (in en). U.S. National Library of Medicine. https://medlineplus.gov/druginfo/meds/a601121.html.
- ↑ 17.0 17.1 "Certain 4-substituted amino-2,1,3-benzothiadiozoles" US patent 3843668, published 1974-10-22, assigned to Sandoz AG
- ↑ "Research on 2,1,3-thia- and selenadiazole". Chemistry of Heterocyclic Compounds 3 (5): 662–666. 1969. doi:10.1007/BF00468340.
- ↑ "Pharmaceutical Substances: Tizanidine". Thieme. https://pharmaceutical-substances.thieme.com/ps/search-results?docUri=KD-20-0115.
External links
- "Tizanidine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tizanidine.
- "Tizanidine hydrochloride". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/tizanidine%20hydrochloride.
- "Tizanidine side effects". Drug Information Portal. tizanidine-side-effects.us. https://tizanidine-side-effects.us/.
 |