Chemistry:Glibornuride
From HandWiki
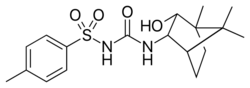
| |
| Names | |
|---|---|
| IUPAC name
N-{[(1R,2R,3S,4S)-2-Hydroxybornan-3-yl]carbamoyl}-4-methylbenzene-1-sulfonamide
| |
| Systematic IUPAC name
N-{[(1S,2S,3R,4R)-3-Hydroxy-4,7,7-trimethylbicyclo[2.2.1]heptan-2-yl]carbamoyl}-4-methylbenzene-1-sulfonamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
| MeSH | C073323 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H26N2O4S | |
| Molar mass | 366.48 g/mol |
| Pharmacology | |
| 1=ATC code }} | A10BB04 (WHO) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Glibornuride (INN) is an anti-diabetic drug from the group of sulfonylureas.[1] It is manufactured by MEDA Pharma and sold in Switzerland under the brand name Glutril.[2]
Synthesis
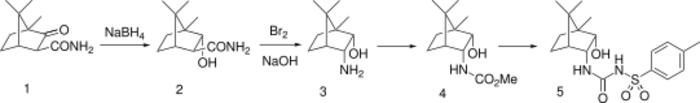
Glibornuride synthesis:[3] See also: U.S. Patent 3,770,761; eidem, U.S. Patent 3,654,357 (to Hoffmann-La Roche).
Gliburnide is an endo-endo derivative made from camphor-3-carboxamide by borohydride reduction (exo approach), followed by Hofmann rearrangement to carbamate, followed by displacement with sodium tosylamide.
References
- ↑ "Pharmacodynamic aspects of tolbutamide, glibenclamide, glibornuride and glisoxepide. I. Dose response relations and repeated administration in diabetic subjects". Diabetologia 7 (6): 449–54. December 1971. doi:10.1007/bf01212061. PMID 5004178.
- ↑ "Glutril — Drugs.com". https://www.drugs.com/international/glutril.html.
- ↑ Bretschneider, H.; Hohenlohe-Oehringen, K.; Graßmayr, K. (1969). "Arylsulfonylureido- und Arylsulfonylamidoacyl-derivate von Oxy- und Oxo-cycloalkanen als potentielle Antidiabetica". Monatshefte für Chemie 100 (6): 2133. doi:10.1007/BF01151769.
 |

