Chemistry:AM-938
From HandWiki
Short description: Chemical compound
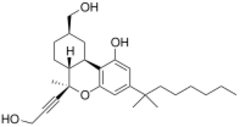 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C27H40O4 |
| Molar mass | 428.613 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
AM-938 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid receptor agonist. It is a derivative of HU-210 which has been substituted with a 6β-(3-hydroxyprop-1-ynyl) group. This adds a "southern" aliphatic hydroxyl group to the molecule as seen in the CP-series of nonclassical cannabinoid drugs, and so AM-938 represents a hybrid structure between the classical and nonclassical cannabinoid families,[1] with the 6-hydroxyalkyl chain rigidified with a triple bond. This gives AM-938 a greater degree of selectivity, so while it is still a potent agonist at both CB1 and CB2, it is reasonably selective for CB2, with a Ki of 0.3nM at CB2 and 1.2nM at CB1, a selectivity of around 4x.[2][3]
See also
- AM-4030 - double bond instead of a triple bond
- AM-919 - saturated rather than a triple bond
- HU-243 - "southern" side chain replaced by methyl unit
References
- ↑ Pertwee, Roger. Cannabinoids. Handbook of Experimental Pharmacology. 168. Springer. p. 269. ISBN 3-540-22565-X.
- ↑ "Classical/non-classical cannabinoid hybrids; stereochemical requirements for the southern hydroxyalkyl chain". Life Sciences 56 (23–24): 2007–12. 1995. doi:10.1016/0024-3205(95)00182-6. PMID 7776825.
- ↑ "Classical/nonclassical hybrid cannabinoids: southern aliphatic chain-functionalized C-6beta methyl, ethyl, and propyl analogues". Journal of Medicinal Chemistry 41 (19): 3596–608. September 1998. doi:10.1021/jm960677q. PMID 9733485.
 |

