Chemistry:AM-2389
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C25H38O3 |
| Molar mass | 386.576 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
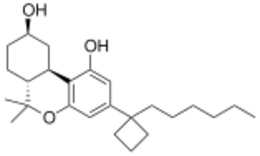
AM-2389 is a classical cannabinoid derivative which acts as a potent and reasonably selective agonist for the CB1 receptor, with a Ki of 0.16 nM, and 26× selectivity over the related CB2 receptor. It has high potency in animal tests of cannabinoid activity, and a medium duration of action.[1][2] Replacing the 1',1'-dimethyl substitution of the dimethylheptyl side chain of classical cannabinoids with cyclopropyl or cyclopentyl results in higher potency than cyclobutyl, but only the cyclobutyl derivatives show selectivity for CB1 over CB2.[3] High selectivity for CB1 over CB2 is difficult to achieve (cf. AM-906, AM-1235), as almost all commonly used CB1 agonists have similar or greater affinity for CB2 than CB1, and the only truly highly selective CB1 agonists known as of 2012 are eicosanoid derivatives such as O-1812.[citation needed]
See also
References
- ↑ "Novel 1',1'-chain substituted hexahydrocannabinols: 9β-hydroxy-3-(1-hexyl-cyclobut-1-yl)-hexahydrocannabinol (AM2389) a highly potent cannabinoid receptor 1 (CB1) agonist". Journal of Medicinal Chemistry 53 (19): 6996–7010. October 2010. doi:10.1021/jm100641g. PMID 20925434.
- ↑ "AM2389, a high-affinity, in vivo potent CB1-receptor-selective cannabinergic ligand as evidenced by drug discrimination in rats and hypothermia testing in mice". Psychopharmacology 220 (2): 417–26. March 2012. doi:10.1007/s00213-011-2491-1. PMID 21989802.
- ↑ "C1'-cycloalkyl side chain pharmacophore in tetrahydrocannabinols". Journal of Medicinal Chemistry 50 (17): 4048–60. August 2007. doi:10.1021/jm070121a. PMID 17672444.
 |
