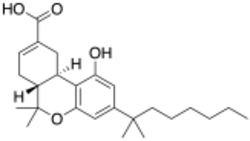
Chemistry:Ajulemic acid
 | |
| Clinical data | |
|---|---|
| Trade names | Lenabasum |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Minimal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H36O4 |
| Molar mass | 400.559 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ajulemic acid (1',1'-Dimethylheptyl-delta-8-tetrahydrocannabinol-11-oic acid) (DMH-D8-THC-11-OIC) (AB-III-56, HU-239, IP-751, CPL 7075, CT-3, JBT-101, Anabasum, Resunab, Lenabasum) is a synthetic cannabinoid that shows anti-fibrotic and anti-inflammatory effects in pre-clinical studies without causing a subjective "high".[1] Although its design was inspired by a metabolite of delta-9-THC known as delta-9-THC-11-oic acid, ajulemic acid is an analog of the delta-8-THC metabolite delta-8-THC-11-oic acid.[2][3] It is being developed for the treatment of inflammatory and fibrotic conditions such as systemic sclerosis, dermatomyositis and cystic fibrosis.[4] It does not share the anti-emetic effects of some other cannabinoids, but may be useful for treating chronic inflammatory conditions where inflammation fails to resolve.[5] Side effects include dry mouth, tiredness, and dizziness. The mechanism of action is through activation of the CB2 receptor leading to production of specialized proresolving eicosanoids such as lipoxin A4 and Prostaglandin J2. Studies in animals at doses up to 40 mg/kg show minimal psychoactivity of ajulemic acid, compared to that produced by tetrahydrocannabinol.[6] A composition of ajulemic acid named Lenabasum (formerly Anabasum, Resunab) is being developed by Corbus Pharmaceuticals (formerly JB Therapeutics) for the treatment of orphan chronic life-threatening inflammatory diseases.[7][citation needed]
References
- ↑ "Ajulemic acid: A novel cannabinoid produces analgesia without a "high"". Life Sciences 75 (12): 1513–1522. August 2004. doi:10.1016/j.lfs.2004.04.010. PMID 15240185.
- ↑ "Cannabimimetic properties of ajulemic acid". The Journal of Pharmacology and Experimental Therapeutics 320 (2): 678–686. February 2007. doi:10.1124/jpet.106.111625. PMID 17105826.
- ↑ "Potent Anti-Inflammatory and Pro-Resolving Effects of Anabasum in a Human Model of Self-Resolving Acute Inflammation". Clinical Pharmacology and Therapeutics 104 (4): 675–686. October 2018. doi:10.1002/cpt.980. PMID 29238967.
- ↑ "Effect of the cannabinoid ajulemic acid on rat models of neuropathic and inflammatory pain". Neuroscience Letters 382 (3): 231–235. July 2005. doi:10.1016/j.neulet.2005.03.019. PMID 15925096.
- ↑ "Ajulemic acid (IP-751): synthesis, proof of principle, toxicity studies, and clinical trials". The AAPS Journal 7 (1): E143–E148. June 2005. doi:10.1208/aapsj070115. PMID 16146336.
- ↑ "Cannabimimetic properties of ajulemic acid". The Journal of Pharmacology and Experimental Therapeutics 320 (2): 678–686. February 2007. doi:10.1124/jpet.106.111625. PMID 17105826.
- ↑ "Companies To Watch: Corbus Pharmaceuticals". https://www.lifescienceleader.com/doc/corbus-pharmaceuticals-0001.
