Chemistry:AMG-3
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
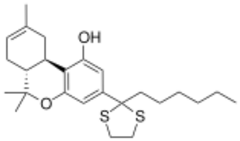
| Formula | C25H36O2S2 |
| Molar mass | 432.68 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
AMG-3 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ8-THC substituted with a dithiolane group on the 3-position side chain.[1] AMG-3 is a potent agonist at both CB1 and CB2 receptors with a Ki of 0.32 nM at CB1 and 0.52 nM at CB2,[2][3] and its particularly high binding affinity has led to it being used as a template for further structural development of novel cannabinoid drugs.[4] It has sedative and analgesic effects, with analgesia lasting for up to 36 hours after administration.[5]
See also
References
- ↑ "Structure elucidation and conformational properties of synthetic cannabinoids (-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hydroxy-6H-dibe nzo [b,d]pyranyl)-2-hexyl-1,3-dithiolane and its methylated analog". Journal of Pharmaceutical and Biomedical Analysis 18 (6): 947–56. January 1999. doi:10.1016/s0731-7085(98)00100-9. PMID 9925329.
- ↑ "Pharmacophoric requirements for cannabinoid side chains: multiple bond and C1'-substituted delta 8-tetrahydrocannabinols". Journal of Medicinal Chemistry 41 (7): 1195–200. March 1998. doi:10.1021/jm970277i. PMID 9544219.
- ↑ "Pharmacophoric requirements for the cannabinoid side chain. Probing the cannabinoid receptor subsite at C1'". Journal of Medicinal Chemistry 46 (15): 3221–9. July 2003. doi:10.1021/jm020558c. PMID 12852753.
- ↑ "Combined 3D QSAR and molecular docking studies to reveal novel cannabinoid ligands with optimum binding activity". Bioorganic & Medicinal Chemistry Letters 17 (24): 6754–63. December 2007. doi:10.1016/j.bmcl.2007.10.044. PMID 17980589.
- ↑ "Behavioral pharmacological properties of a novel cannabinoid 1',1'-dithiolane delta8-THC analog, AMG-3". Behavioural Pharmacology 16 (5–6): 499–510. September 2005. doi:10.1097/00008877-200509000-00024. PMID 16148456.
External links
 |
