Chemistry:XLR-12
From HandWiki
Short description: Chemical compound
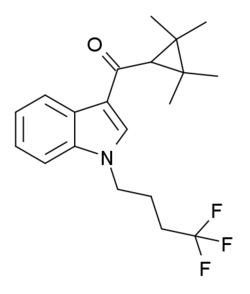 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H24F3NO |
| Molar mass | 351.413 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
XLR-12 is an indole-based synthetic cannabinoid drug that was invented by Abbott Laboratories in 2006.[1] It is an analogue of XLR-11 where the 5-fluoropentyl chain has been replaced with a 4,4,4-trifluorobutyl chain. XLR-12 is relatively highly selective for the CB2 receptor, with a Ki of 0.09 nM and 167x selectivity over the related CB1 receptor, however it still retains appreciable affinity for CB1 with a Ki of 15 nM.[2]
Legal status
XLR-12 is illegal in Hungary[3] and Japan.[4]
See also
References
- ↑ Pace JM, Tietje K, Dart MJ, Meyer MD, "3-Cycloalkylcarbonyl indoles as cannabinoid receptor ligands", WO patent application 2006069196, published 2006-06-29, assigned to Abbott Laboratories
- ↑ "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". Journal of Medicinal Chemistry 53 (1): 295–315. January 2010. doi:10.1021/jm901214q. PMID 19921781.
- ↑ A Magyarországon megjelent, a Kábítószer és Kábítószer-függőség Európai Megfigyelő Központjának Korai Jelzőrendszerébe (EMCDDA EWS) 2005 óta bejelentett ellenőrzött anyagok büntetőjogi vonatkozású besorolása
- ↑ "指定薬物名称・構造式一覧(平成27年9月16日現在)" (in Japanese). 厚生労働省. 16 September 2015. http://www.mhlw.go.jp/seisakunitsuite/bunya/kenkou_iryou/iyakuhin/yakubuturanyou/dl/meisho.pdf.
 |
