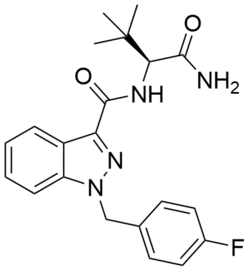
Chemistry:ADB-FUBINACA
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C21H23FN4O2 |
| Molar mass | 382.439 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
ADB-FUBINACA (ADMB-FUBINACA[1]) is a designer drug identified in synthetic cannabis blends in Japan in 2013.[2][3] In 2018, it was the third-most common synthetic cannabinoid identified in drugs seized by the Drug Enforcement Administration.[4]
The (S)-enantiomer of ADB-FUBINACA is described in a 2009 Pfizer patent[5] and has been reported to be a potent agonist of the CB1 receptor and the CB2 receptor with EC50 values of 1.2 nM and 3.5 nM, respectively.[5][6] ADB-FUBINACA features a carboxamide group at the 3-indazole position, like SDB-001 and STS-135. ADB-FUBINACA appears to be the product of rational drug design, since it differs from AB-FUBINACA only by the replacement of the isopropyl group with a tert-butyl group.
An analogue of ADB-FUBINACA, ADSB-FUB-187, containing a more functionalized carboxamide substituent was recently reported.
Side effects
One death through coronary arterial thrombosis has been linked to ADB-FUBINACA intoxication.[7]
Metabolism
Twenty-three ADB-FUBINACA major metabolites were identified in several incubations with cryopreserved human hepatocytes. Major metabolic pathways were alkyl and indazole hydroxylation, terminal amide hydrolysis, subsequent glucuronide conjugations, and dehydrogenation.[8]
Legality
In the United States, ADB-FUBINACA is a Schedule I controlled substance.[9]
See also
References
- ↑ Pulver, Benedikt; Fischmann, Svenja; Gallegos, Ana; Christie, Rachel (March 2023). "EMCDDA framework and practical guidance for naming synthetic cannabinoids". Drug Testing and Analysis 15 (3): 255–276. doi:10.1002/dta.3403.
- ↑ "Two new-type cannabimimetic quinolinyl carboxylates, QUPIC and QUCHIC, two new cannabimimetic carboxamide derivatives, ADB-FUBINACA and ADBICA, and five synthetic cannabinoids detected with a thiophene derivative α-PVT and an opioid receptor agonist AH-7921 identified in illegal products". Forensic Toxicology 31 (2): 223–240. July 2013. doi:10.1007/s11419-013-0182-9.
- ↑ "Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications". Pharmaceuticals (Basel, Switzerland) 14 (3): 186. February 2021. doi:10.3390/ph14030186. PMID 33669071.
- ↑ "Emerging Threat Report: Annual 2018". Special Testing and Research Laboratory, Drug Enforcement Administration. https://ndews.umd.edu/sites/ndews.umd.edu/files/Emerging-Threat-Report-2018-Annual.pdf.
- ↑ 5.0 5.1 "Indazole Derivatives" WO patent 2009106982
- ↑ "Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA". ACS Chemical Neuroscience 6 (9): 1546–59. September 2015. doi:10.1021/acschemneuro.5b00112. PMID 26134475.
- ↑ "Death Associated With the Use of the Synthetic Cannabinoid ADB-FUBINACA". Journal of Analytical Toxicology 40 (3): 236–9. April 2016. doi:10.1093/jat/bkv142. PMID 26755539.
- ↑ "In Vitro Metabolite Profiling of ADB-FUBINACA, A New Synthetic Cannabinoid". Current Neuropharmacology 15 (5): 682–691. July 2017. doi:10.2174/1570159X15666161108123419. PMID 29403341.
- ↑ "Schedules of Controlled Substances: Temporary Placement of Six Synthetic Cannabinoids (5F-ADB, 5F-AMB, 5F-APINACA, ADB-FUBINACA, MDMB-CHMICA and MDMB-FUBINACA) Into Schedule I". Drug Enforcement Administration. https://www.deadiversion.usdoj.gov/fed_regs/rules/2016/fr1221.htm.
 |
